Methods for the Preparation of Enolates
1. Deprotonation

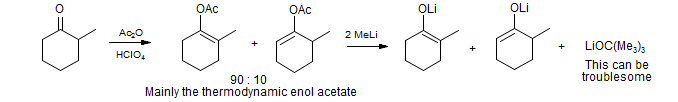
2. Cleavage of Enol Acetates
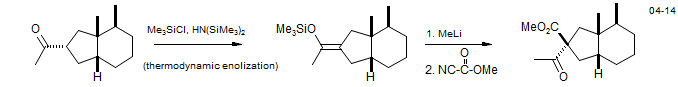
3. Cleavage of Enol Silyl Ethers
Isopetasol: Bohlmann, F.; Otto, W. Ann. Chem. 1982, 186
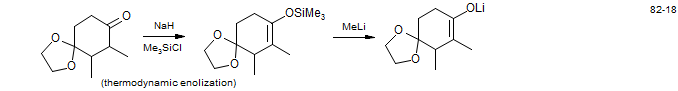
Bakkenolide-A: Reddy, D. S.. Org. Lett. 2004, 6, 3345
4. Conjugate Addition to α,β-Unsaturated Carbonyl Compounds
Trifarienols A and B: Huang, Forsyth, J. Org. Chem. 1995, 60, 5746.
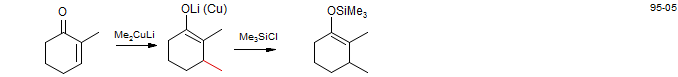
5. Conjugate Reduction of α,β-Unsaturated carbonyl Compounds
Classically, the lithium-ammonia reaction of enolates was used to prepare solutions of enolates from α,-β-unsaturated ketones.
Ferruginol: Snitman, D. L.; Himmelsbach, R. J.; Watt, D. S. J. Org. Chem., 1978, 43, 4758.
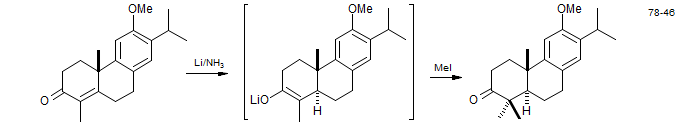
Upial: Taschner, M. J.; Shahripour, A. J. Am. Chem. Soc. 1985, 107, 5570.
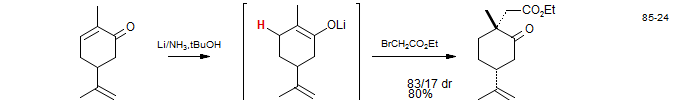
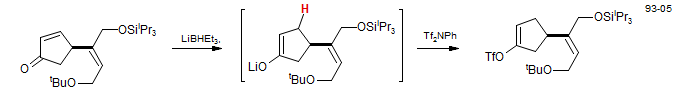
Lithium triethylborohydride shows an unusual proclivity for conjugate reduction (1,4 addition), and such reactions can be used to form enolates. Strychnine: Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1993, 115, 9293-9294; Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1995, 117, 5776- 5788.