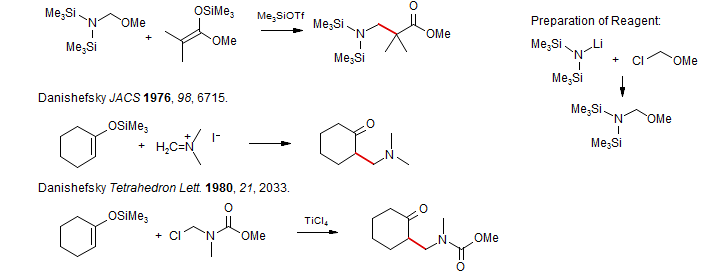
Carbonium Ion Alkylation of Enol Silyl Ethers
Enol Silyl Ethers: "O-Silylated Enolates--Versatile Intermediates for Organic Synthesis," Rasmussen, J. K. Synthesis 1977, 91. "Enol Ethers in Synthesis," Brownbridge, P. Synthesis 1983, 1, 85. "Cross-Coupling Reactions Based on Acetals," Mukaiyama, T.; Murakami, M. Synthesis 1987, 1043. "Synthesis and Reactions of Functionalized Silyl Enol Ethers," Poirier, J. M. Org. Prep. & Proc. Int'l. 1988, 20, 317. "Palladium-mediated Arylation of Enol Ethers," Daves, G. D. Jr. Adv. in Metal-Organic Chemistry. Vol 2, 1991, Jai Press: Greenwich CT. "Alkoxide-Mediated Preparation of Enolates from Silyl Enol Ethers and Enol Acetates-From Discovery to Synthetic Applications." Cahard, D.; Duhamel, P. Europ. J. Org. Chem. 2001, 1023-31.
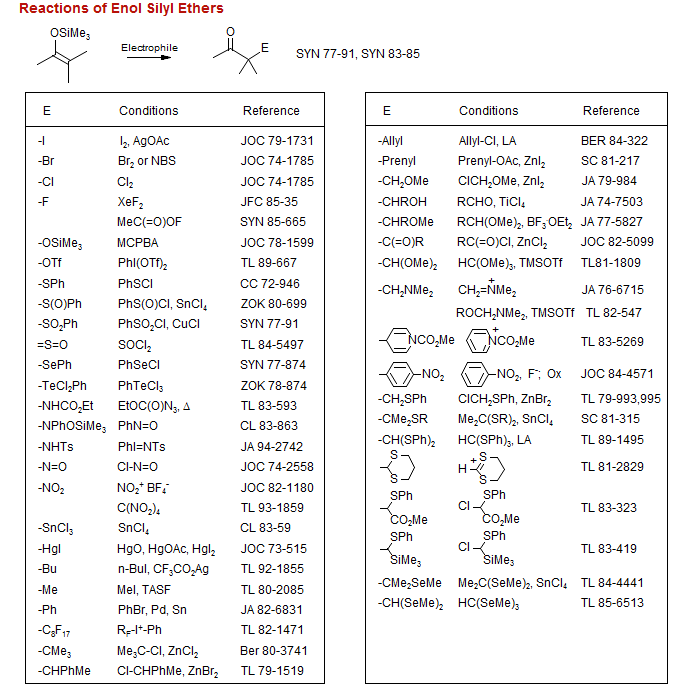
Enol silyl ethers can be cleaved with nucleophiles such as MeLi, LiNH2 or R4N+F- to give reactive enolates. They can also be used directly as weak nucleophiles with very reactive electrophiles such as carbonium ions, the halogens (Br2, Cl2, I2) or pseudohalogens (PhSCl, PhSeCl, Cl-N=O).
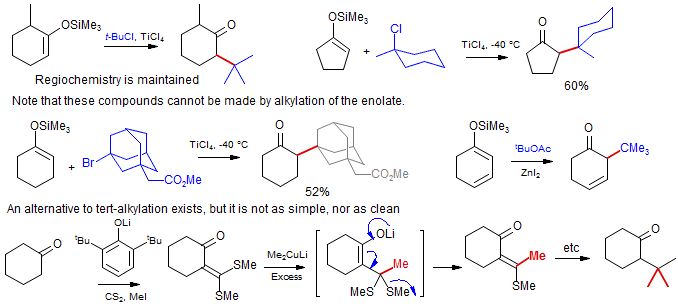
• tert-Alkylation of Enol Ethers. It is difficult to introduce t-alkyl groups α to ketones. Ebol ethers react with tert carbopnium ions to form such compounds. The method is not used much in practice. (Reetz Chem. Ber. 1980, 113, 3734; Angew. Chem. Int. Ed. 1982, 21, 96; Chan Tetrahedron Lett. 1977, 4183).
• Benzylation (Patterson, Tetrahedron Lett., 1979, 1519).
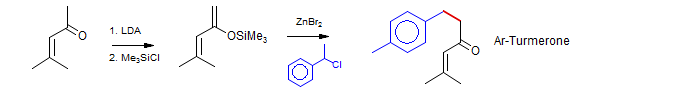
• γ-Alkylation of Dienol Silyl Ethers. Ketone and ester dienolates usually react predominantly at the α position (Schlessinger, Tet. Lett. 1973, 2433): e.g. Kozikowski and Stein, J. Am. Chem. Soc. 1982, 104, 4023
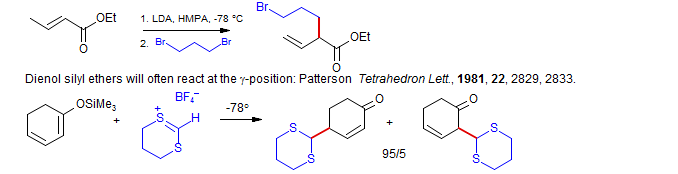
Evans, J. Am. Chem. Soc., 1993, 115, 4497.
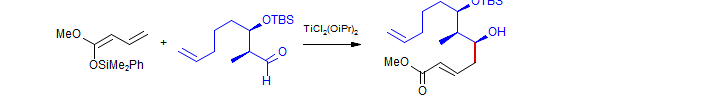
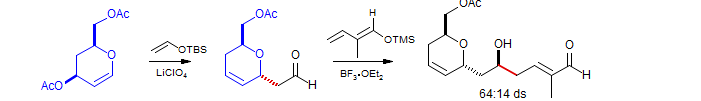
Grieco, Tet. Lett., 1998, 39, 1275.
• Aminomethylation (Okano, Morimoto, Sekiya, Chem. Commun., 1984, 883).