Conjugate Addition - the Michael and Related Reactions
An asymmetric version is used in the synthesis of Strychnine: Ohshima, T.; Xu, Y.; Takita, R.; Shimizu, S. Zhong, D.; Shibasaki, M. J. Am. Chem. Soc. 2002, 124, 14546-14547. DOI
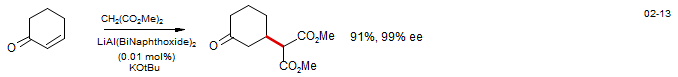
Conjugate Addition: "Stereochemistry of the Base-promoted Michael Addition Reaction," Oare, D. C.; Heathcock, C. H. Top. Stereochem. 1989, 19, 227. "Synthesis of Anthracyclinones by the Electrophilic and Nucleophilic Addition to Anthraquinones," Krohn, K. Tetrahedron 1990, 46, 291. "Acyclic Stereocontrol in Michael Addition Reactions of Enamines and Enol Ethers," Oare, D. C.; Heathcock, C. H. Topics in Stereochem. 1991, 20, 87. "Is the Transition State Indeed intermediate Between Reactants and Products? The Michael Addition Reaction as a Case Study," Hoz, S. Acc. Chem. Res. 1993, 26, 69. "Syntheses of Polycyclic Natural Products Employing the Intramolecular Double Michael Reaction," Masataka, I.; Fukumoto, K. Angew. Chem. I. E. Engl. 1993, 32, 1010. "Intramolecular Michael and anti-Michael Additions to Carbon-Carbon Triple Bonds," Rudorf, W. D.; Schwarz, R. Synlett 1993, 369. "Control of Asymmetry through Conjugate Addition Reactions," Leonard, J. Contemporary Org. Synth. 1994, 1, 387-416. "The Intramolecular Michael Reaction," Little, R. D.; Masjedizadeh, M. R.; Wallquist, O.; McLoughlin, J. I. Org. React. 1995, 47, 315-552. "Recent Advances in Catalytic Enantioselective Michael Additions," Krause, N.; Hoffmann-Roder, A. Synthesis 2001, 171-96. "Construction of Quaternary Stereocenters: New Perspectives through Enantio-selective Michael Reactions," Christoffers, J.; Baro, A. Angew. Chem. Int. Ed. Engl. 2003, 42, 1688-90. Recent Advances in Asymmetric Organocatalytic 1,4-Conjugate Additions." Tsogoeva, S. B. Europ. J. Org. Chem. 2007, 1701-16. "Recent Advances in Metal-catalyzed Asymmetric Conjugate Additions." Christoffers, J.; Koripelly, G.; Rosiak, A.; Roessle, M. Synthesis 2007, 1279-300. "Organocatalytic Asymmetric Conjugate Additions." Almasi, D.; Alonso, D. A.; Najera, C. Tetrahedron: Asymmetry 2007, 18, 299-365.
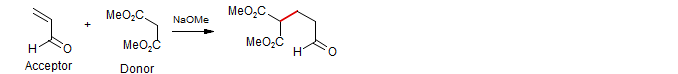
Many kinds of alkenes substituted with electron withdrawing groups undergo facile addition reactions with nucleophiles. Successful use of such conjugate additions requires that several potential pitfalls be circumvented:
1. A clear distinction must be made between the donor (an enol or enolate) and acceptor (the α,β-unsaturated carbonyl compound). In the classical Michael condensation, the donor (β-dicarbonyl compound) is much more acidic than the acceptor (an enone), so with a weak base only the β-dicarbonyl compound is significantly converted to enolate.
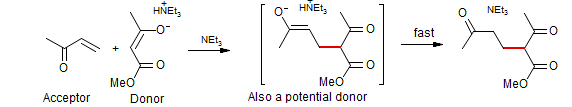
2. The product of a conjugate addition is itself a potential donor, and reaction of the product donor with the acceptor must be controlled to avoid Michael polymerization. In the classical Michael condensation the product enolate is much more basic than the donor enolate,and is thus rapidly discharged by protonation by solvent, other proton donors (e.g., by HNEt3+ if NEt3 is used as base) or by the starting β-dicarbonyl compound..
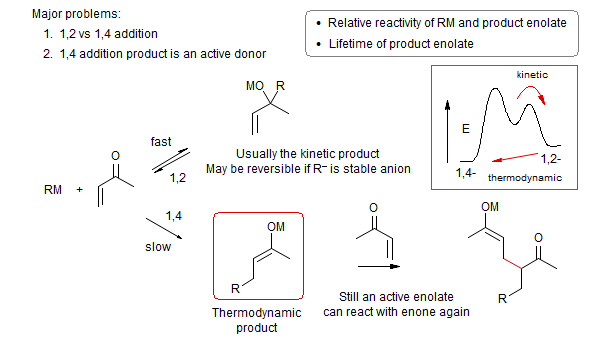
3. Many acceptors can react either in a 1,2 (undesired) or 1,4 fashion (desired). There are two principal ways this regioselectivity can be controlled. The 1,4 adduct is almost always thermodynamically more stable, so selecting conditions where the 1,2-addition is reversible will result in formation of 1,4 products. This is the situation in the classical Michael condensations shown above. Alternatively, 1,4 additions can sometimes be achieved by choosing a proper base/donor/acceptor/solvent combination that favors 1,4 addition kinetically.
Schultz J. Org. Chem. 1976, 41, 4044
Most successful Michael additions fall into one of three general categories which use different strategies for minimizing Michael polymerization:
1. Weak donor which can be generated at relatively low pH, under conditions where the product enolate (which typically has a much higher pKa than the donor) is rapidly protonated. Such reactions are usually run under reversible conditions (thermodynamic control), using protic solvents or amine bases. Often only a catalytic amount of base is used since the product enolate regenerates the basic catalyst.
Under these conditions the 1,2-additions are highly reversible, and usually do not accumulate in the reaction mixture. The classical Michael reaction (β-dicarbonyl, cyano-carbonyl, β-ketosulfoxides, etc as donors) are of this type, as are reactions using nitroalkanes, thiolates, amines, cyanide ion and other weakly basic nucleophiles as donors.
For some reactions of this type the conjugate addition may also be quite reversible, and may even have an unfavorable equilibrium. For the latter type the final protonation is needed to shift the equilibrium towards products.
Tetrahedron Lett. 1976, 4793
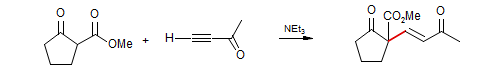
Parker J. Org. Chem. 1980, 45, 1218

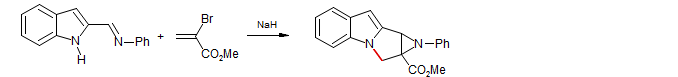
2. Reactions in which polymerization is avoided by discharge of the 1,4 product enolate by some chemical reaction, most commonly a β-elimination, but other reactions (such as the Wittig cyclization below) have also been used. Since the enolate cannot build up in the reaction mixture, such reactions do not need to have proton donors present. In fact, proton donors may be deleterious to subsequent transformations of the product enolate.
Dionne Can. J. Chem. 1978, 56, 419
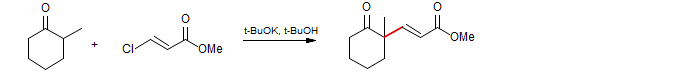
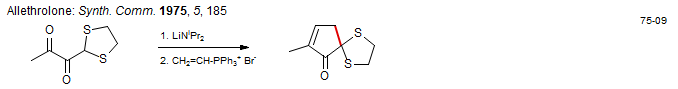
Cory Chem. Commun. 1983, 1244
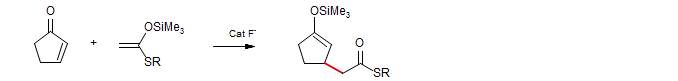
The fluoride-catalyzed addition of enol silyl ethers and silyl ketene acetals can be considered to be of this type. Here the product enolate is passivated by silylation.
Gerlach Helv. Chim. Acta 1978, 61, 2503
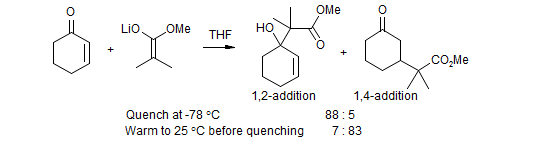
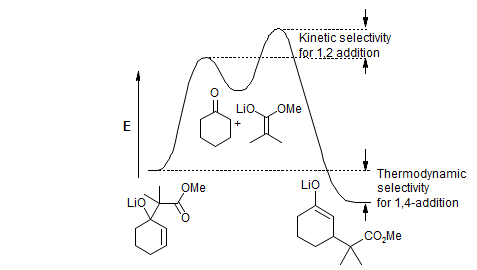
3. Reactions in which the product enolate, for steric and/or electronic reasons, is less reactive than the donor, and hence does not compete effectively with the donor. Such reactions will typically be run under aprotic conditions, often with kinetic control of enolization and addition. The product enolate is present in stoichiometric amounts at the end of the reaction, and can be used for subsequent transformations (conjugate addition--alkylation).
In some reactions of this type the 1,2-addition may be reversible (such as in the cyclohexenone plus ester enolate example above), and conjugate addition products can be obtained if equilibration is allowed to go to completion. Generally, if the pKa of the donor is well under 30, then 1,2 and sometimes even 1,4 additions can be made reversible if sufficiently polar solvents or high enough temperatures are used.
For more basic nucleophiles both the 1,2- and 1,4-additions are irreversible, and the reaction will only give useful products if conditions or reagents can be found that give minimal 1,2 addition. The reactions of most organolithium, Grignard and organocopper reagents are of this type.
Methyl vinyl ketone analog - Isopetasol: Bohlmann, Otto Ann. Chem. 1982, 186; Stork J. Am. Chem. Soc. 1973, 95, 6152
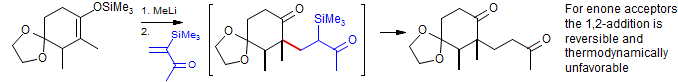
Schlessinger J. Am. Chem. Soc. 1974, 96, 3702
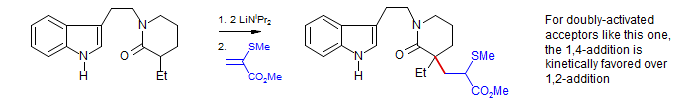
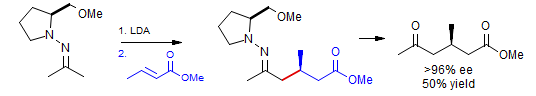
Asymmetric Michael Addition: Enders, Papadopoulos Tet. Lett. 1983, 24, 4967
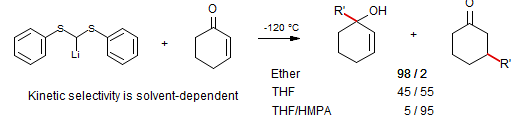
Reich, Sikorski J. Org. Chem. 1999, 64, 14; Sikorski, Reich J. Am. Chem. Soc. 2001, 123, 6527
Alkyl, vinyl and aryl organocopper reagents show a high selectivity for 1,4 addition to enones, and are among the most widely used reagents for this purpose.
Asymmetric Conjugate Addition,
Rossiter, B. E.; Swingle, N. M. Chem. Rev. 1992, 92, 771.v
The Asymmetric Michael Addition Reaction Using Chiral Imines,
d'Angelo, J.; Desmaële, D.; Dumas, F.; Guingant, A. Tetrahedron: Asymmetry 1992, 3, 459.