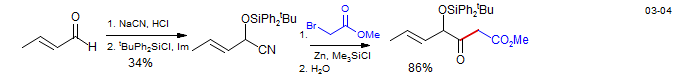The Aldol Reaction and Condensation
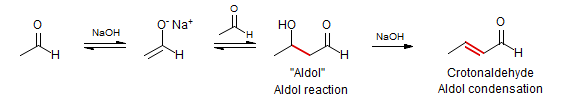
The reaction of carbonyl compound enolates with aldehydes and ketones to form a β-hydroxy carbonyl compound is the Aldol Reaction, if conditions result in a subsequent dehydration to form the α,β-unsaturated compound, then the reaction is termed the Aldol Condensation (loss of a molecule of water). These reactions represent a very large and complex area of chemistry. Chemists have developed techniques to successfully convert almost all possible combinations of donors (the enolate) and acceptors (the aldehyde or ketone) to aldol products. Crossed aldol reactions in which the acceptor is an aromatic aldehyde are sometimes called Claisen-Schmidt Condensations.
Aldol Reactions under equilibrating conditions
To run successful aldol reactions under equilibrating conditions requires either that the donor and acceptor be the same, or that there be some molecular features that allow the donor and acceptor components to be distinguished. The self-aldolization of aldehydes can usually be stopped at the aldol stage, with more vigorous conditions (or a separate dehydration step) needed to form the enal.
The self-aldolization of ketones is usually slow, and the equilibrium constant for aldol formation is often unfavorable. Only after dehydration does the reaction become thermodynamically favorable.
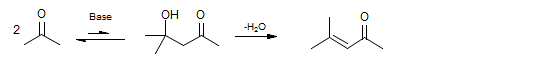
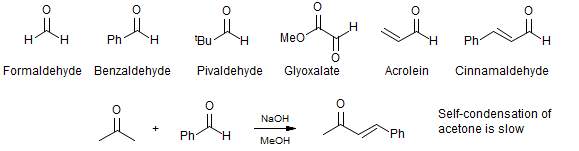
Crossed aldol reactions in which the acceptor cannot enolize are often successful. Some acceptors of this type are:
Intramolecular Aldol
Intramolecular aldol reactions, particularly those where there is no confusion about the donor and acceptor as in the examples below, often work quite well under equilibrating conditions. Strained rings (3, 4, 8, 9 membered) can't usually be formed, but reactions which form five and 6-membered rings are common. Quadrone: Takeda, K.; Shimono, Y.; Yoshii, E. J. Am. Chem. Soc. 1983, 105, 563.
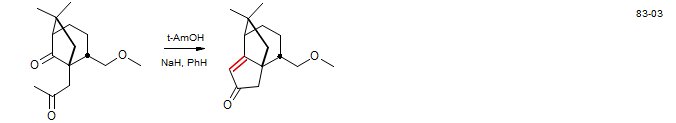
Dichroanone: McFadden, R. M.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 7738

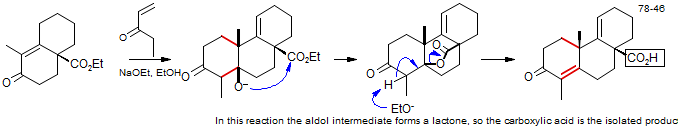
The Robinson Annulation involves a Michael addition of an enolate (typically of a 1,3-dicarbonyl compound) to form a 1,5-dicarbonyl compound. These conjugate additions are successful because the 1,2-addition of the enolates to the enone is highly reversible, and usually has an unfavorable equilibrium. In some cases this step is performed separately, but often continued treatment by base leads to an intramolecular aldol condensation to form the final product, a cyclohexenone, as in the example below: Synthesis of Ferruginol: Snitman, D. L.; Himmelsbach, R. J.; Watt, D. S. J. Org. Chem., 1978, 43, 4758.
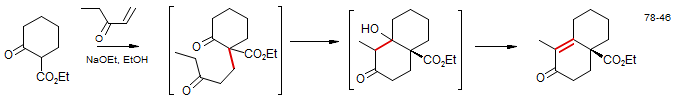
In this synthesis of Ferruginol, a second Robinson annulation was performed on the enone product. Note that under these equilibrating conditions it is the conjugated enolate (not the cross-conjugated one) that reacts.
A more sophisticated Robinson annulation is shown below. Here the much more acidic cyclic 1,3-diketone is the preferred Michael donor, allowing a successful conjugate addition. Acanthoic Acid: Ling, T.; Chowdhury, C.; Kramer, B. A.; Vong, B. G.; Palladino, M. A.; Theodorakis, E. A. J. Org. Chem. 2001, 66, 8843. DOI
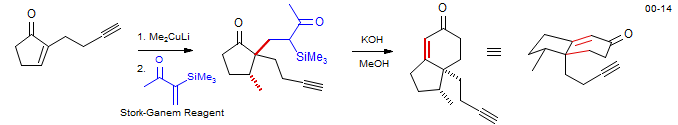
Robinson annulations can also be initiated by the reaction of preformed enolates with Michael acceptors. However, reactions with methyl vinyl ketone are often unsuccessful, because the enolate which is formed in the conjugate addition is very similar in reactivity to the starting enolate. The α-trimethylsilylvinyl methyl ketone introduced by Stork (sometimes called the Stork-Ganem Reagent - J. Am. Chem. Soc. 1973, 95, 6152.) solves this problem - the trimethylsilyl-substituted enolate product is more hindered, and the anion somewhat stabilized by silicon, so a second conjugate additions (polymerization) is inhibited. In the example below the enolate is formed by conjugate addition of an organocuprate. The silyl group is readily removed during the hydroxide catalyzed aldol reaction. Hispidospermidine: Frontier, A. J.; Raghavan, S.; Danishevsky, S. J. J. Am. Chem. Soc. 2000, 122, 6151. DOI
The Directed Aldol Reaction
A much more general approach to controlling aldol reactions is to use preformed enolates or enol derivatives. Lithium enolates of ketones, esters, lactones and amides usually react cleanly with aldehyde substrates. Ester, amide and carboxylate enolates also react well with ketones to form aldol products, but intermolecular ketone-ketone crossed aldols are generally unsuccessful with alkali metal enolates, as are most aldol reactions involving aldehyde enolate donors.
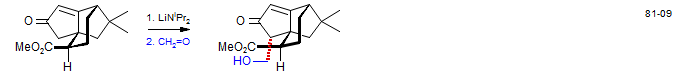
Quadrone: Danishefsky, S.; Vaughan, K.; Gadwood, R. C.; Tsuzuki, K. J. Am. Chem. Soc. 1981, 103, 4136.
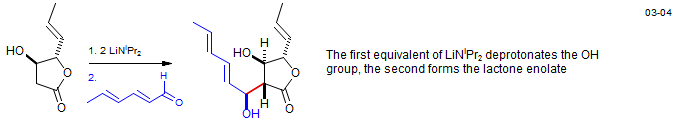
Aldol reaction of a β-hydroxylactone - Waol A: Gao, X.; Nakadai, M.; Snider, B. Org. Lett. 2003, 5, 451
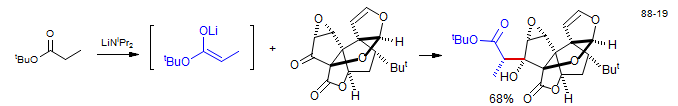
Aldol reaction with ester enolate: synthesis of Ginkolide B: Corey, E. J.; Kang, M. C.; Desai, M. C.; Ghosh, A. K.; Houpis, I. N. J. Am. Chem. Soc. 1988, 110, 649-651.
Crossed Aldol Reactions using Metalloenamines
Metaloenamines (azaenolates) can be used for those crossed Aldol Reactions (ketone-donor/ketone-acceptor and aldehyde-donor) that cannot be done using preformed lithium enolates.
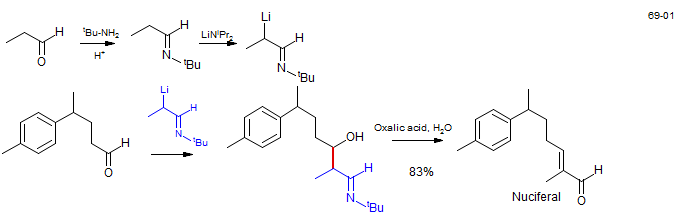
Aldehyde crossed aldol - synthesis of Nuciferal: Büchi, G.; Wüest, H. J. Org. Chem. 1969, 34, 1122
Crossed Aldol Reactions using Enol Silyl Ethers (Mukaiyama Aldol)
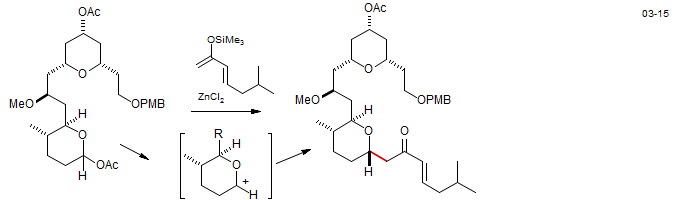
Mukaiyama aldol reactions are Lewis acid catalyzed (the acceptor is an oxy-substituted carbonium ion, the donor an enol silyl ether). Psymberin: Rech, J. C.; Floreancig, P. E. Org. Lett. 2005, 7, 5175-5178. DOI
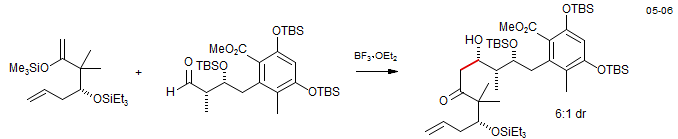
The acceptor can be Lewis acid complex of an aldehyde or ketone (as in the example above) or an oxycarbonium ion formed by ionization of an acetal or ketal. Leucascandrolide A: Williams, D. R.; Patnaik, S.; Plummer, S. V. Org. Lett. 2003, 5, 5035. DOI
Crossed Aldol Reactions using the Reformatsky Reagent
The Reformatsky reaction involves the reduction of an α-halo carbonyl compound (usually a bromo ester) with zinc to form a zinc enolate, which condenses with an adehyde or ketone. Classically the reaction was performed under Barbier conditions (ketone, halide and zinc mixed), but separate preparation of the reagent has some significant advantages.
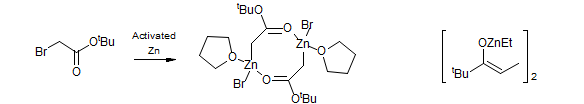
The X-ray structure of a Reformatsky reagent has been reported (Dekker, J. Chem. Commun. 1983, 553). Both carbon and oxygen are bonded to zinc. Ketone zinc enolates seem to be O-zincated (Hansen, M. M.; Bartlett, P. A.; Heathcock, C. H. Organometallics., 1987, 6, 2069)
The Reformatsky Reaction,
Rathke, M. W. Org. React. 1975, 22, 423.
Shriner, R. L. Org. React. 1942, 1, 1.
Recent Advances in the Reformatsky Reactions,
Furstner, A. Synthesis 1989, 571
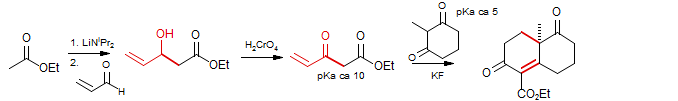
Reformatsky reactions with γ-bromocrotonates can give products from either α or γ reaction, depending on reaction conditions (Hudlicky, J. Org. Chem. 1984, 49, 1845). In the example below (synthesis of Abscisic Acid), the γ-adduct was obtained. Presumably the 1,2-addition was reversible under these conditions. Constantino, M. G.; Losco, P.; Castellano, E. E. J. Org. Chem. 1989, 54, 681. DOI
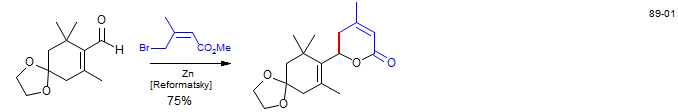
Reformatsky reaction on a nitrile - Synthesis of Waol A: Gao, X.; Nakadai, M.; Snider, B. Org. Lett. 2003, 5, 451