The Acylation of Enolates - the Claisen and Dieckmann Condensations
The classical Claisen condensation involves the reaction of an ester with an ester enolate to form a β-keto ester.
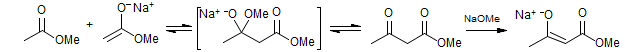
The reactions of ketone enolates with carboxylate or carbonate esters to give 1,3-diketones, ketoaldehydes or ketoesters are also usually referred to as Claisen Condensations. In fact these are used much more frequently than the classical ester plus ester enolate version.
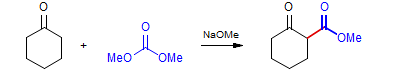
The Claisen Condensation differs from enolate alkylation and the aldol reaction in that the product often has a much more acidic α-hydrogen than does the starting ester or ketone (pKa of ketoester is 8-11, of MeOH is 16, and of esters is >22). Thus proton transfers between product and starting material are almost inevitable. For this reason the enolization is usually done with a stoichiometric amount of base (often MeONa or EtONa in MeOH or EtOH, depending on which ester is being used). The final step of the reaction is made irreversible by deprotonation of the β-keto ester, which is substantially more acidic (pKa in DMSO 14) than the starting ester (pKa in DMSO 29) or the conjugate acid of the alkoxide. If the final product cannot be deprotonated to form the stabilized enolate, then the Claisen condensation under equilibrating conditions usually fails because the process is thermodynamically unfavorable.
Several strategies have been developed for successful acylation of enolates:
1. Claisen condensations in which donor and acceptor are the same.
This strategy is used for the large-scale synthesis of simple β-keto esters and β-diketones (like methyl acetoacetate itself), but is not generally useful. A rare example in complex molecule synthesis is shown below
Coccinelline: Stevens, R. V.; Lee, A. W. N. J. Am. Chem. Soc. 1979, 101, 7032. DOI
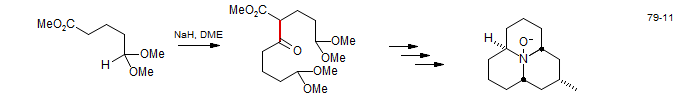
2. Intramolecular Acylation - the Dieckmann Condensation
An intramolecular Claisen condensation of a diester is called a Dieckmann condensation. Often the esters of symmetric diacids are used to avoid regioselectivity problems.
Isonootkatone: Marshall, J. A.; Faubl, H.; Warne, T. M. Chem. Commun. 1967, 753
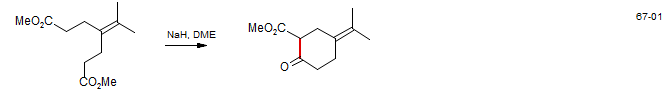
β-Elemenone. Here a one-pot Dieckmann condensation - alkylation is performed: Majetich, G.; Grieco, P. A.; Nishizawa, N. J. Org. Chem. 1977, 42, 2327.
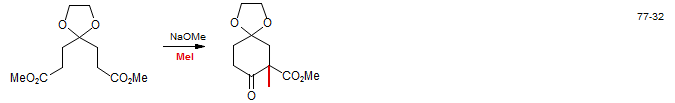
3. Enolization under thermodynamic control using non-enolizable acylating agents.
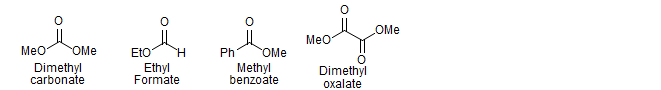
The most commonly used acceptors are carbonate and formate esters. The donor can be either a ketone or ester enolate.
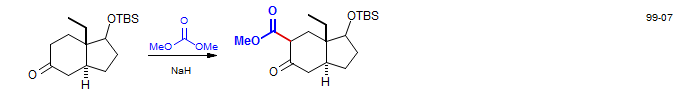
Desogestrel: Corey, E. J.; Huang, A. X. J. Am. Chem. Soc. 1999, 121, 710
The reaction of ketones with formate esters under base catalysis gives keto-aldehydes (sometimes called hydroxymethylene ketones because they exist largely in the enolic form. Warburganal: Kende, A. S.; Blacklock, T. J. Tetrahedron Lett. 1980, 21, 3119
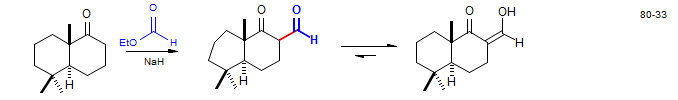
4. Kinetic enolization followed by C-acylation
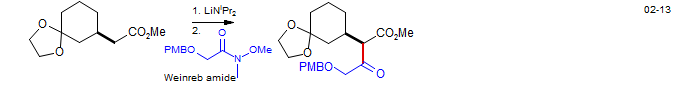
The acylation of preformed enolates with reactive acylating agents like Cl-CO2Me or acyl chlorides usually gives an unsatisfactory mixture of O- and C-acylation. However, several acylating agents with a high propensity for C-acylation under kinetic conditions have been found. The most commonly used is the Mander reagent (methyl cyanoformate: Mander and Sethi, Tetrahedron Lett. 1983, 24, 5425) which reliably acylates ketone enolates under conditions of kinetic control. Weinreb reagents (N-methoxy amides) are also sometimes successsful.
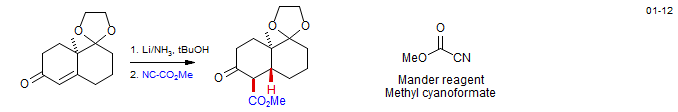
The Mander reagent allows the formation of β-ketoesters with quaternary centers, which cannot be done with Claisen condensations under thermodynamic control, since the final enolization step cannot occur. In the example below, the thermodynamic enolate is prepared by silylation, the silyl enol ether converted to enolate and then trapped with the Mander reagent. Synthesis of Bakkenolide: Reddy, D. S.. Org. Lett. 2004, 6, 3345. DOI
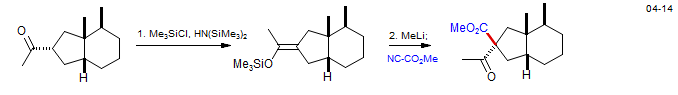
Utilization of a Weinreb amide to acylate an ester enolate - Strychnine: Ohshima, T.; Xu, Y.; Takita, R.; Shimizu, S. Zhong, D.; Shibasaki, M. J. Am. Chem. Soc. 2002, 124, 14546-14547
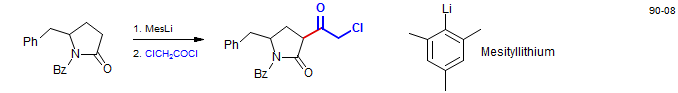
Acid chlorides can sometimes be used in enolate acylations, but the presence of diisopropylamine (from the LiNiPr2) can cause problems. In the example below the deprotonation was carried out with mesityllithium to give an amine-free enolate solution. Zygosporin E - Vedejs, E.; Reid, J. G.; Rodgers, J. D.; Wittenberger, S. J. J. Am. Chem. Soc. 1990, 112, 4351-4357. Vedejs, E.; Wittenberger, S. J. J. Am. Chem. Soc. 1990, 112, 4357-4364