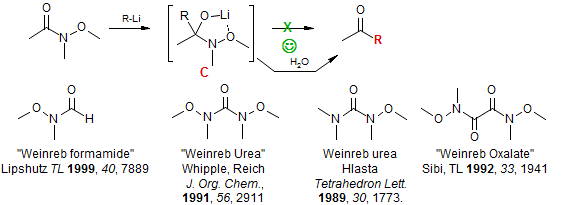
The Acylation of Organometallic Reagents
The reaction of organometallic reagents with carboxylic esters, acid halides and other carboxylic acid derivatives is an important method for the formation of ketones. These reactions can be complicated by the relatively high reactivity of the product ketones towards organometallic reagents, which make double additions to form tertiary alcohols hard to avoid completely. Apparently in these reactions the tetrahedral intermediate A decomposes to the ketone during the reaction, leading to significant double addition products B
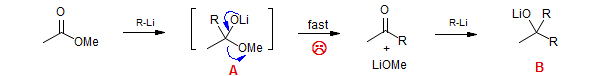
One general solution to this problem is the use of special amides as acylation agents, the most useful of which have turned out to be N-methoxy amides, often termed "Weinreb amides" (Nahm, S.; Weinreb, S. M. Tetrahedron Lett. 1981, 22, 3815). The double addition is avoided here because the tetrahedral intermediate C, is stable (the amide nitrogen is a poor leaving group), and the ketone (or aldehyde, if a hydride donor is used) is not released until the reaction is worked up by treatment with water.
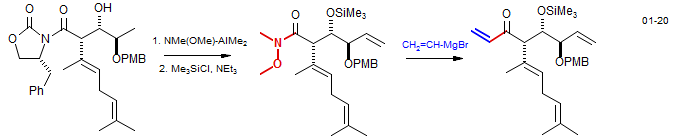
A typical application of a Weinreb ester is shown below as part of the synthesis of Fumagillol: Boiteau, J.-G.; Van de Weghe, P.; Eustache, J. Org. Lett.. 2001, 3, 2737. DOI
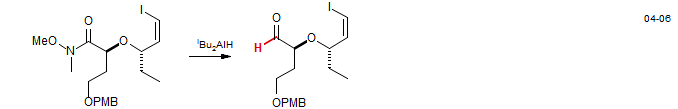
Weinreb amides are also frequently used to convert carboxylic acid derivatives to aldehydes by reaction with hydride donors like LiAlH4 and diisobutylaluminum hydride. From the synthesis of Brasilenyne: Denmark, S.; Yang, S. M. J. Am. Chem. Soc. 2004, 126, 12432 DOI.
Morpholino amides will also work: Scheidt, K. A. Org. Lett., 2004, 6, 3977