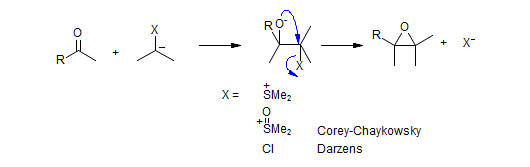α-X Carbon Nucleophiles - Methylenation and Epoxidation Reagents
There is a family of carbon nucleophiles with an α-substituent which helps to stabilize the negative charge, and which is involved in subsequent transformations. When such nucleophiles are added to ketones and aldehydes, the intermediate alkoxide interacts with the X-substituent in several ways: X can be either an electrophile, which results in olefination reactions, or act as a leaving group, which results in epoxide formation.
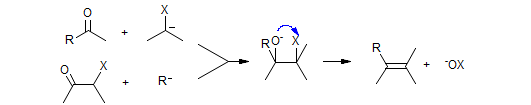
X =
PPh3 (Wittig)
P(O)R2 (Horner-Wadsworth-Emmons)
SiMe3 (Peterson)
SO2Ar (Julia)
SePh (Krief-Reich)
Cr (Takai)
Ti/Al (Tebbe, Petasis)
Ti/Zn (Nozaki-Takai)