Methylenation with Phosphorus Ylids - Wittig Reaction.
The reactions of α-phosphorus nucleophiles with carbonyl compounds are reliable methods for the synthesis of olefins. There are two principal variants: the Wittig Reaction, which uses phosphonium ylids as nucleophiles, and the Horner-Wadsworth-Emmons reaction, which uses metalated phosphonates.
"The Wittig Reaction", Maercker, A. Org. Synth. 1965, 14, 270. "Cycloalkenes by Intramolecular Wittig Reaction," Beeker, K. B. Tetrahedron 1980, 36, 1717. "The Wittig Olefination Reaction of Carbonyl Compounds Other than Aldehydes and Ketones," Murphy, P. J. Chem. Soc. Rev. 1988, 17, 1. "The Wittig Olefination Reaction and Modifications Involving Phosphoryl-stabilized Carbanions. Stereochemistry, Mechanism, and Selected Synthetic Aspects," Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863. "Synthesis of Heterocycles by the aza-Wittig Reaction," Gusar, N.I. Russ. Chem. Rev. 1991, 60, 146. "Stereochemistry and mechanism in the Wittig reaction," Vedejs, E.; Peterson, M. J. Top. Stereochemistry 1994, 21, 1. "Recent Synthetic Applications of the Non-Classical Wittig Reaction," Murphy, P. J.; Lee, S. E. JCS Perk. Trans I 1999, 3049-66.
Nobel-Prize-winning chemistry
G. Wittig, 1979
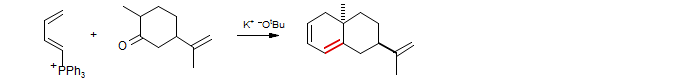
The parent Wittig reagent, methylenetriphenylphosphorane, is the most commonly used one, even though there are now a number of effective alternative reagents for methylenation of ketones (Tebbe reagent, Takai-Lombardo). The double-Wittig example below is from Ficini, J.; Revial, G.; Genet, J. P. Tetrahedron Lett. 1981, 22, 629, 633. DOI
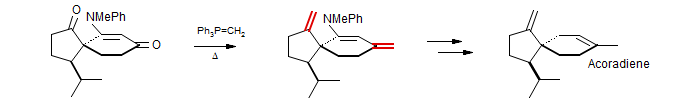
Wittig reagents bearing one alkyl substituent on the ylidic carbon have a strong tendency to form cis olefins (>90% in many cases, if proper reaction conditions are used). The example below is from a synthesis of Argentilactone (Carretero, J. J.; Ghosez, L Tetrahedron Lett. 1988, 29, 2059. DOI) Also commonly used are aryl and vinyl-substituted ylids, for which mixtures of cis and trans olefins are typically seen.
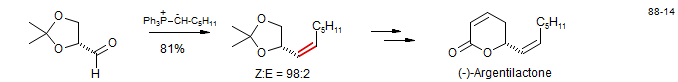
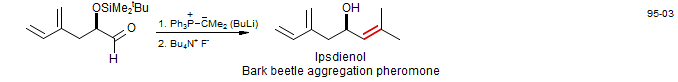
Dialkyl-substituted Wittig reagents react poorly or not at all with enolizable ketones, but aldehydes usually work well. (Terada, M.; Mikami, K. Chem. Commun. 1995, 2391 DOI).
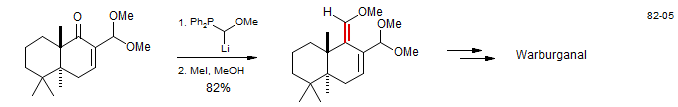
Homologation of Aldehydes with Methoxymethylenephosphorane
A common method for the conversion of aldehydes to the next higher homolog utilizes Ph3P=CHOMe (MacMillan, D. W. C.; Overman, L. E. J. Am. Chem. Soc. 1995, 117, 10391 DOI).
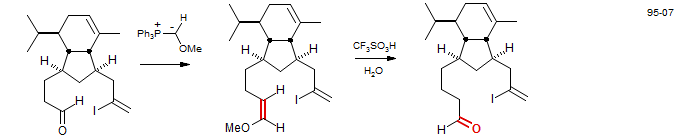
In sterically hindered cases, the Wittig reaction fails. An alternative uses Ph2PCHLiOMe, which is much more reactive and less sterically hindered than the Wittig reagent. After carbonyl addition of the lithium reagent, the phosphonium salt is generated by alkylation with MeI (Wender. P. A.; Eck, S. L Tetrahedron Lett. 1982, 23, 1871 DOI)
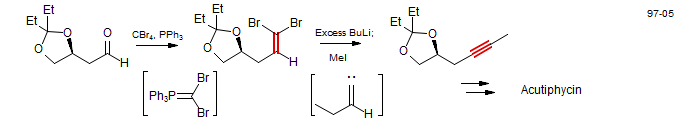
Corey-Fuchs Reaction - Synthesis of Acetylenes from Aldehydes
The Corey-Fuchs reaction produces 1,1-dibromoalkenes, which are commonly converted to alkynes by treatment with butyllithium (the Fritsch-Buttenberg-Wiechell rearrangement). The lithium acetylides can be used directly for subseqent reactions, if needed. (Smith, A. B.; Chen, S. S.-Y.; Nelsom, F.; Reichert, J. M.; Salvatore, B. A. J. Am. Chem. Soc. 1997, 119, 10935. DOI).
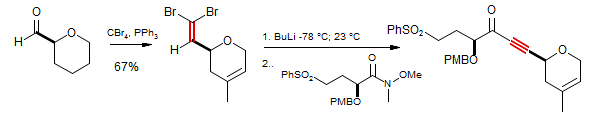
Synthesis of (-)-Laulimalide (Gosh, Wang, Kim J. Org. Chem. 2001, 66, 8973)
Alkylation of Wittig Reagents.
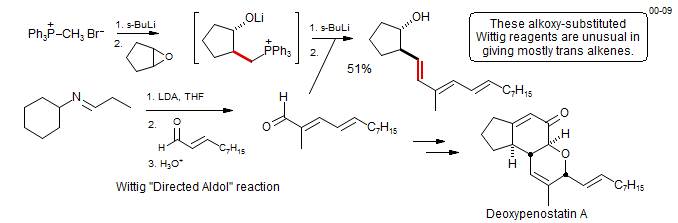
Wittig reagents are sufficiently nucleophilic that alkylations can be performed to form more complex reagents. An example from the synthesis of Deoxypenostatin A (Snider, B. B.; Liu, T. J. Org. Chem. 2000, 65, 8490-8498. DOI).
Stabilized Wittig Reagents
Carbonyl-stabilized Wittig Reagents react only with aldehydes. Trans products are usually obtained. Pumiliotoxin B: Lin, N.-H.; Overman, L. E.; Rabinowitz, M. H.; Robinson, L. A.; Sharp, M. J.; Zablock, J.. J. Am. Chem. Soc. 1996, 118, 9062. DOI
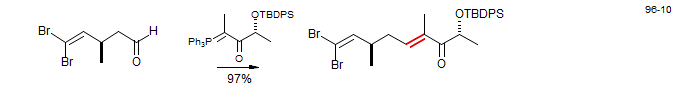
Below an example of introducing the Evans chiral auxiliary by a Wittig reaction (D. A. Evans, W. C. Black J. Am. Chem. Soc. 1993, 115, 4497).
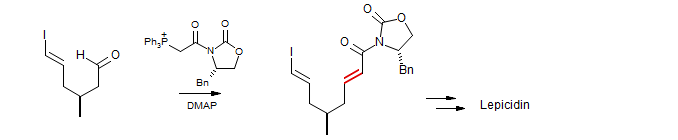
Conjugate Addition to Vinylphosphonium Salts
Addition of nucleophiles to vinylphosphonium salts produces Wittig reagents, which can be captured by carbonyl electrophiles (Kawamoto, Muramatsu, Yura Synth. Comm. 1975, 5, 185). Write mechanisms for the reactions below:
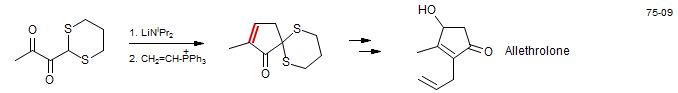
Intramolecular Wittig Reactions. J. Org. Chem. 1975, 40, 100