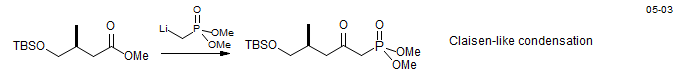
Horner-Wadsworth-Emmons Reaction
"Olefin Synthesis with Organic Phosphonate Carbanions," J. Boutagy and R. Thomas, Chem. Rev. 1974, 74, 87. "Synthetic Applications of Phosphoryl Stabilized Anions," W. S. Wadsworth, Jr. Org. React. 1977, 25, 73. "Wadsworth-Emmons Reaction Revisited," W. J. Stec, Acc. Chem. Res. 1983, 16, 411. "Vinylphosphonates in Organic Synthesis," Minami, T.; Motoyoshiya, J. Synthesis 1992, 333. Minami, T.; Okauchi, T.; Kouno, R. α-Phosphonovinyl Carbanions in Organic Synthesis. Synthesis 2001, 349-57. "Stereocontrol in Organic Synthesis Using the Diphenylphosphoryl Group," Clayden, J.; Warren, S. Angew. Chem. Int. Ed. Engl. 1996, 35, 241-70.
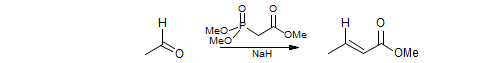
The HWE reaction is the reaction of a carbonyl compound with an α-metalated phosphonate to give an alkene. The reaction is usually used when the nucleophilic carbon bears a strong anion stabilizing group (CO2Me, COMe, COH, CN, SO2R, SOR, vinyl, phenyl). Like the analogous Wittig reagents, the metalated phosphonates tend to give trans olefins if the substituents on phosphorus are simple alkoxy groups, and if lithium or sodium counterions are used. They can become cis-selective if non-coordinating cations (e.g., K+ - 18-crown-6) or electron withdrawing substituents on the phosphonate ester groups are used (e.g. CF3CH2O- or ArO).
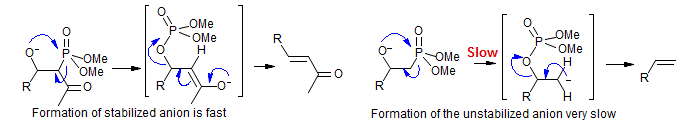
In the absence of a carbanion-stabilizing group on the anionic carbon the elimination reaction to form the double bond becomes very slow. Apparently the transition state for the syn-elimination resembles the carbanion formed by cleavage of the P-C bond:
Metalated phosphonates are substantially more reactive than analogous Wittig reagents, and will react with ketones as well as aldehydes. This can be predicted from the much higher basicity of phosphonates. Stabilized Wittig reagents react only with aldehydes.
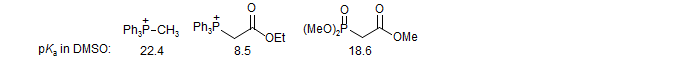
Preparation of Phosphonates
Simple keto- and ester-substituted phosphonates are prepared by the Arbusov reaction - alkylation of a trialkyl phosphite with an alkyl bromide or iodide.

Phosphonates without anion stabilizing groups (such as (MeO)2P(O)CH3) can be metalated with strong bases like LiNiPr2 or n-BuLi, and these add smoothly to aldehydes and ketones to form β-hydroxy phosphonates. Since the elimination to form olefins tends to be very slow, and in some cases cannot be achieved, such reactions are useful for the preparation of more complicated ketophosphonates.
Amphoteronolide B: Masamune; et al Tetrahedron Lett. 1988, 29, 451
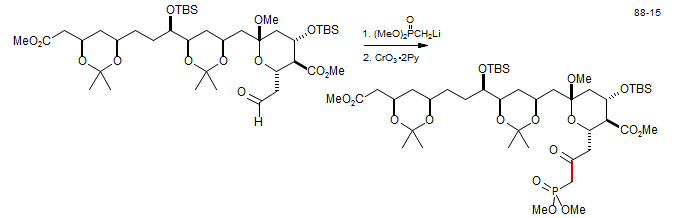
(MeO)2P(O)CH2Li will also react with carboxylic acid derivatives like esters, lactones and amides in a Claisen-like reaction to form keto phosphonates. Dihydroxanthatin: Evans, M. A.; Morken, J. P. Org. Lett. 2005, 7, 3371. DOI
Intramolecular HWE reaction: Heathcock Syn Comm. 1975, 5, 1.
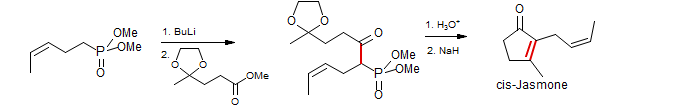
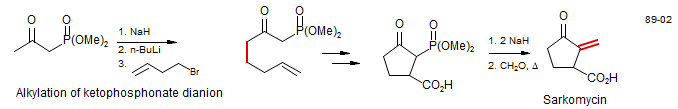
β-Keto phosphonates can be converted to dianions and alkylated at the terminal position, just like β-keto esters: Sarkomycin (Mikolajzyk, M.; Zurawinski, R.; Kielbasinski, P. Tet. Lett. 1989, 30, 1143. DOI)
Juvenile Hormone: Dahm, Trost, Roeller J. Am. Chem. Soc. 1967, 89, 5292.
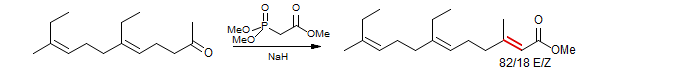
Ionophore Antibiotic X-14547A. Nicolaou, Papahatjis, Claremon, Dolle J. Am. Chem. Soc. 1981, 103, 6967 DOI
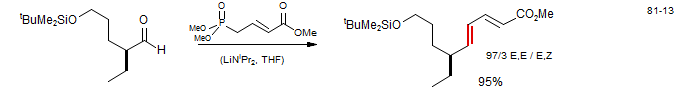
Polyenes with Horner-Wadsworth-Emmons Reagents (Burke, L. T.; Dixon, D. J.; Ley, S. V.; Rodrigues, F. Org. Lett. 2000, 2, 3611. DOI
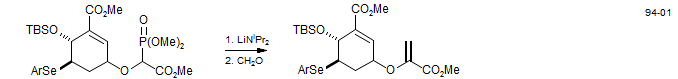
α-Methylenation of Esters - Chorismic acid Synthesis (Mattia, K. M.; Ganem, B. J. Org. Chem. 1994, 59, 720. DOI).
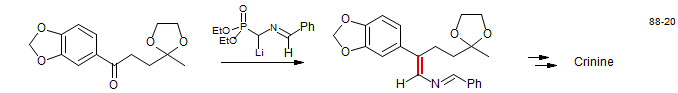
Imine Analog - Synthesis of Crinine (Martin, S. F.; Campbell, C. L. J. Org. Chem. 1988, 53, 3184 DOI).
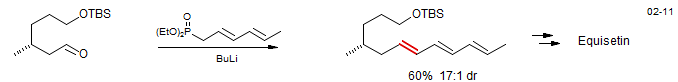
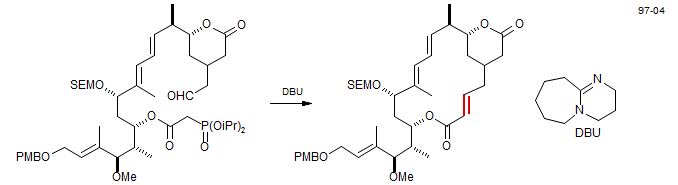
Intramolecular reactions have been successfully used to close large rings. The example below is a key step in the synthesis of Rhyzoxin (D. Williams. D. R.; Werner, K. M.; Feng, B. Tetrahedron Lett. 1997, 38, 6825).
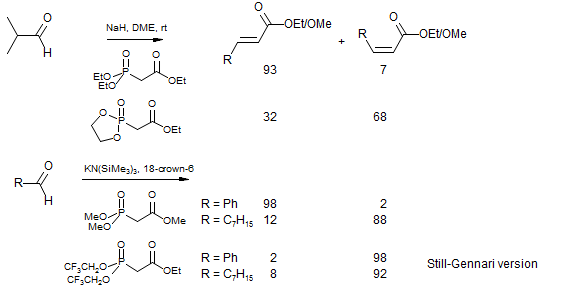
Z-Olefins with Modified Phosphonates
Z α,β-unsaturated carbonyl compounds are formed when phosphonate ester of ethylene glycol (Breuer, Bannet Tetrahedron Lett. 1977, 1141) or trifluoroethanol (Still, Gennari, Tetrahedron Lett. 1983, 24, 4405) are used. Z selectivity is also enhanced if the counterion is rendered weakly electrophilic, (e.g., by using KN(SiMe3)2 / 18-crown-6 as base). In each case it is believed that the switch from E to Z-selectivity is the result of a faster elimination reaction (more reactive alkoxide or more electrophilic phosphorous) which reduces or eliminates the reversibility of the initial addition to the β-alkoxy phosphonate. Presumably the kinetic product initially formed is the streoisomer which produces the Z alkene.
Synthesis of Antillatoxin Isomer (Yamakawa, Shiori J. Org. Chem. 1998, 63, 8638)
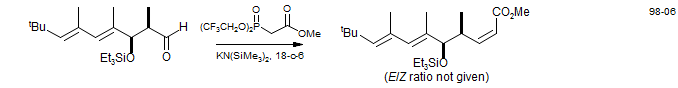
Synthesis of Kallilolide (J. A. Marshall, P. S. Coan J. Org. Chem. 1995, 60, 796)
Substituted Horner-Wadsworth-Emmons Reagents
When there is a substituent on the central carbon of a phosphonate, a trisubstituted alkene will be formed on reaction with an aldehyde. The stereochemical preferences here are complicated, but diligent trial-and-error experiments have revealed that steric effects in the phosphonate can reverse the normal preference for the formation of Z-alkenes, to form predominantly E-alkenes. In the examples below, the alkoxy substituents on both the ester and the phosphonate are progressively increased in size, resulting in a switch from predominantly E to mostly Z stereochemistry (Nagaoka, H.; Kishi, Y. Tetrahedron 1981, 37, 3873).
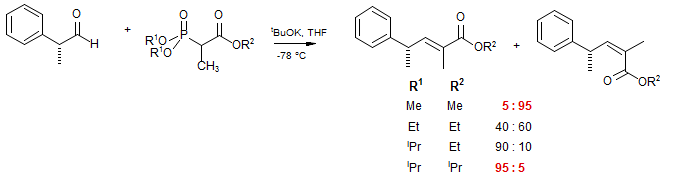
Presumably, here the larger substituents on the phosphonate make the initial carbonyl addition more reversible, leading to a preference for the more stable E-alkene.
Preparation of Acetylenes
There are several methods for the conversion of aldehydes to the homologated acetylenes. One is the Corey-Fuchs Reaction (Ph3P=CBr2 Wittig reagent) followed by dehydrohalogenation as discussed in the Wittig section. The reaction of diazomethylphosphonate (sometimes called the Gilbert-Seyferth reagent: J. Org. Chem. 1982, 47, 1837–1845 DOI) is another commonly used one. Since diazomethylphosphonate is difficult to handle, this reaction is usually done using the Bestmann Reagent (Muller, S.; Liepold, B.; Roth, G. J.; Bestmann, H. J. Synlett 1996, 521), which produces the diazomethylphosphonate in situ by a deacylation reaction (retro-Claisen).
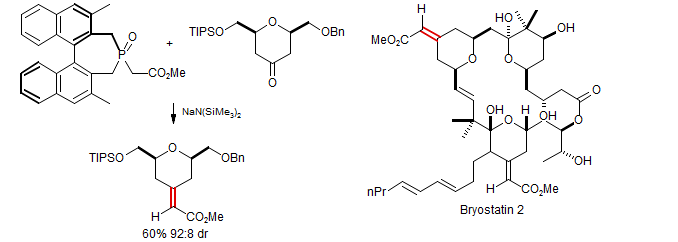
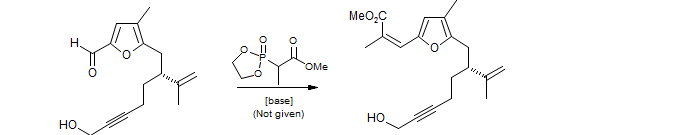
Brenneman, G. B.; Martin, S. F. Org. Lett. 2004, 6, 1329
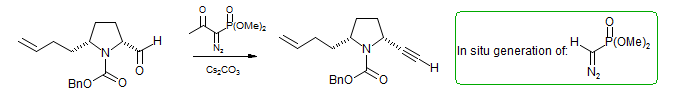
This reaction works by an initial base-catalyzed deacylation of the diazophosphonate, followed by Fritsch-Buttenberg-Wiechell rearrangement of the vinylidene.
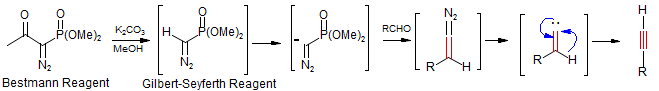
Preparation of allenes with a chiral phosphine oxide (Wittig-Horner Reaction)
Tanaka, Otsubo, Fuji Tetrahedron Lett. 1996, 37, 3735
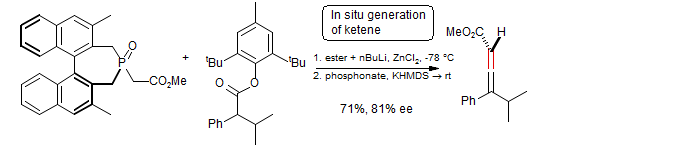
This reagent is also capable of enantiomeric discrimination: Evans, Carter, Carreira, Charette, Prunet, Lautens J. Am. Chem. Soc. 1999, 121, 7540.