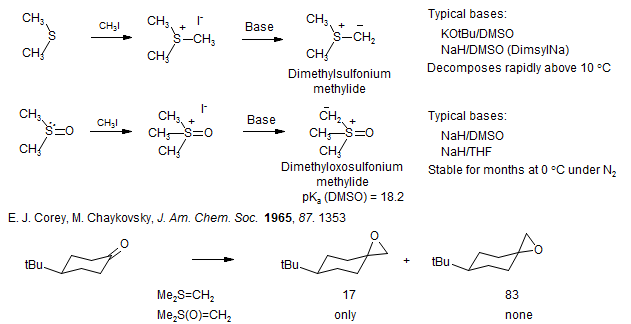
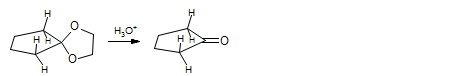Sulfonium Ylid Reactions
Ylid Epoxidation/Cyclopropanation. "Twenty-Five Years of Dimethylsulfoxonium Methylide," Yu. G. Golobov, V. P. Lysenko, I. E. Boldeskul, Tetrahedron 1987, 43, 2609. "Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement," Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2267-340. "Catalytic Asymmetric Epoxidation and Aziridination Mediated by Sulfur Ylides. Evolution of a Project," Aggarwal, V. K. Synlett. 1998, 329-36. "Stereoselective Synthesis of Three-Membered Ring Compounds Via Ylide Routes," Dai, L. X.; Hou, X. L.; Zhou, Y. G. Pure Appl. Chem. 1999, 71, 369. "Catalytic, Asymmetric Sulfur Ylide-Mediated Epoxidation of Carbonyl Compounds: Scope, Selectivity, and Applications in Synthesis," Aggarwal, V. K.; Winn, C. L. Acc. Chem Res. 2004, 37, 611-20.
The Me2SCH2 ylide is believed to be reacting under kinetic control, with axial attack of the ylid favored, leading to the observed major product, the E-epoxide. For the Me2S(O)CH2 ylid, the initial carbonyl addition is reversible, with the more stable equatorial betaine intermediate leading to the only observed product.
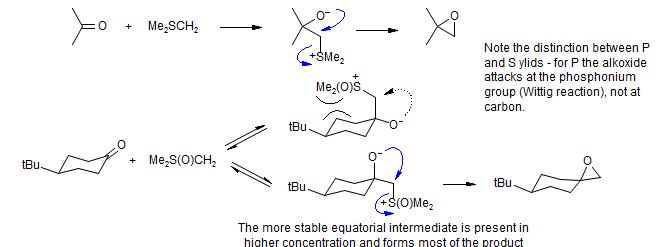
It is also possible that the intermediate from axial attack cyclizes more slowly, since the leaving group has to occupy the congested region above the cyclohexane ring
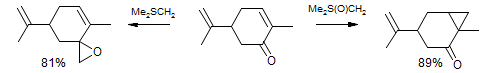
A similar situation obtains for the competition between epoxide formation versus cyclopropanation of enones. The Me2SCH2 ylid irreversibly adds 1,2, leading to epoxide. The 1,2-addition of Me2S(O)CH2 is highly reversible, and the more stable 1,4 adduct leads to the major product.
Felkin Model for Stereochemistry of Nucleophilic Addition to Cyclohexanone
It is experimentally observed that small nucleophiles tend to add to cyclohexanones in an axial fashion, whereas sterically large nucleophiles tend to add equatorially.
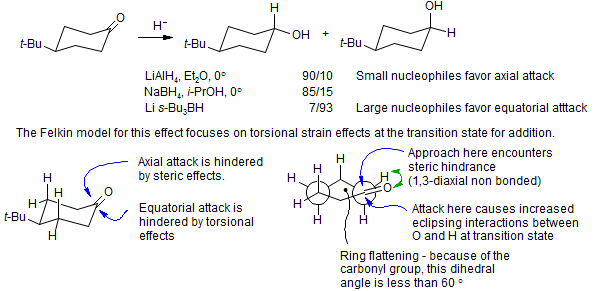
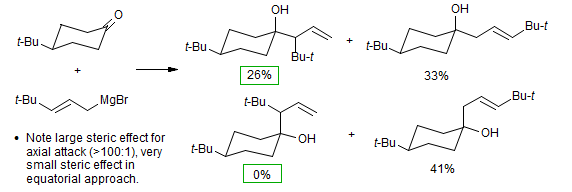
Felkin provided support for this analysis by noting that in the addition of the 3-t-butylallylmagnesium bromide below, there was a significant amount of product from reaction at the more hindered end of the allyl system in the product of equatorial attack, but none detected in the axial attack product (Cherest, M.; Felkin, H. Tetrahedron Lett. 1971, 383-386.).
Similar considerations explain the higher reactivity of cyclohexanones over cyclopentanones in carbonyl additions. In cyclopentanone the C=O interactions with neighboring C-H are largely staggered, but become more eclipsed during the rerduction. In cyclohexanone the C=O and equatorial C-H are almost eclipsed, and become staggered at the transition state for hydride addition.

NaBH4 reduction: k(cyclohexanone) / k(cyclopentanone) = 23 (Brown, H. C.; Ichikawa, K. Tetrahedron 1957, 1, 221.)
Conversely, formation of carbonyl groups is favored in cyclopentanones, with cyclopentanone ethylene ketal hydrolyzing 13 times as fast with acid than cyclohexanone ketal (Conan, J-Y.; Natat, A.; Priolet, D. Bull. Soc. Chim., Fr. 1976, 1935).