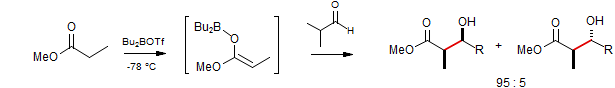The Aldol Condensation - Stereochemistry
Macrolide Antibiotics: Why Chemists are Interested in the Stereochemistry of the Aldol Reaction
Asymmetric Aldol Condensations: "Stereoselective Aldol Condensations," D. A. Evans, J. V. Nelson and T. R. Taber Top. in Stereochem. 1982, 13, 1. "Stereoselective Alkylation Reactions of Chiral Metal Enolates," D. A. Evans in "Asymmetric Synthesis," Vol. 3, J. O. Morrison, Ed., Academic Press, 1984. "Stereoselective Aldol Condensations," C. Heathcock in "Comprehensive Carbanion Chemistry," Part B, E. Buncel, Ed. "Stereoselective Aldol Reactions with α-Unsubstituted Chiral Enolates," M. Braun, Angew. Chem. I. E. 1987, 26, 24. "Recent Development in Stereoselective Aldol Reactions," Braun, M. Adv. Carbanion Chem. Vol 1 1992, Jai Press, Greenwich, CT. "Recent Developments in Asymmetric Aldol Methodology," Franklin, A.S.; Paterson, I. Contemporary Org. Synth. 1994, 1, 317-38. "Catalyzed Enantioselective Aldol Additions of Latent Enolate Equivalents," Nelson, S. G. Tetrahedron: Asymmetry 1998, 9, 357-89. "Asymmetric Catalysis of Aldol Reactions with Chiral Lewis Bases (Trichlorosilyl Enolates)," Denmark, S. E.; Stavenger, R. A. Acc. Chem. Res. 2000, 33, 432-40. "The Catalytic Asymmetric Aldol Reaction." Machajewski, T. D.; Wong, C.-H.; Lerner, R. A. Ang. Chem. IE 2000, 39, 1352-74. "Chiral Enolate Equivalents. A Review," Spino, C. Org. Prep. Proced. Intnl. 2003, 35, 1-140. "Current Progress in the Asymmetric Aldol Addition Reaction," Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Soc, Rev. 2004, 33, 65-75. "Memory of Chirality: Asymmetric Induction Based on the Dynamic Chirality of Enolates," Kawabata, T.; Fuji, K. Top, Stereochem. 2003, 23, 175-205.

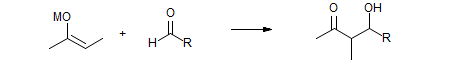
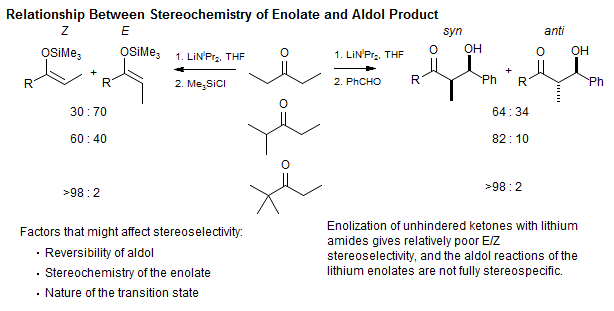
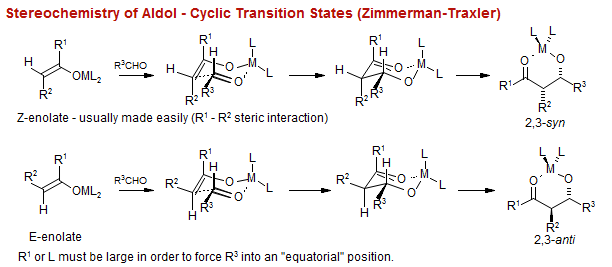
Stereoselection in the Aldol Condensation
W. A. Kleschick, C. T. Buse, C. H. Heathcock, J. Am. Chem. Soc. 1977, 99, 247. Full paper: J. Org. Chem. 1980, 45, 1066.
When the pendant substituent on the ketone is sterically large, then the enolization with lithium amides is highly stereoselective, and the aldol reaction is highly stereospecific (i.e Z enolate gives syn, E enolate gives anti aldol product)
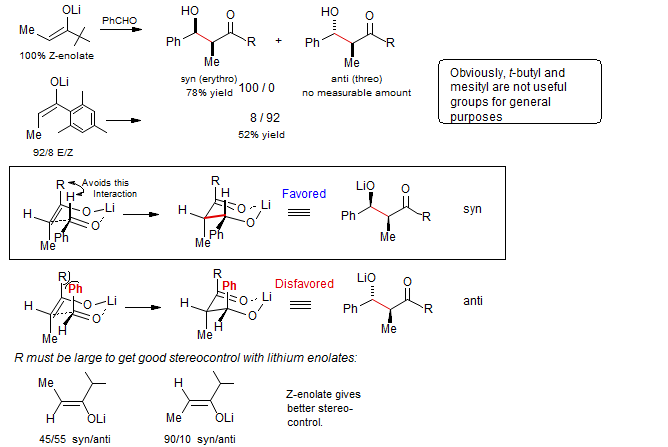
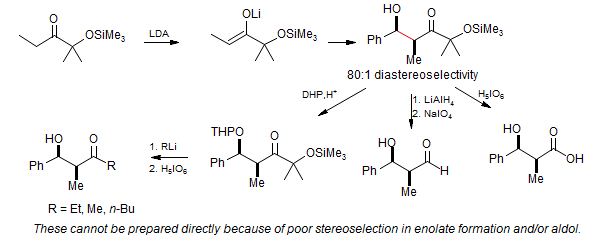
Cleavable "large" groups are more generally useful:
Mesityl and t-butyl groups are not particularly useful for the general synthesis of aldol adducts. However, the introduction of α-oxy substituents preserves the high stereo control of these large groups, and allows a subsequent oxidative cleavage to form useful aldol products. White, Heathcock J.Org.Chem. 1881, 46, 191
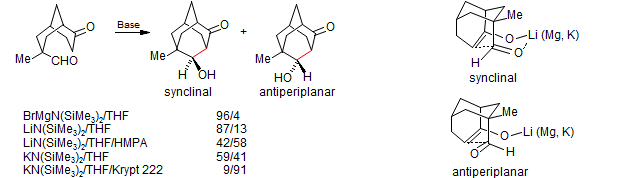
Does the Zimmerman-Traxler Transition State Really Describe the Aldol Condensation?
Denmark, S. E.; Henke, B. R. J. Am. Chem. Soc. 1989, 111, 8032.
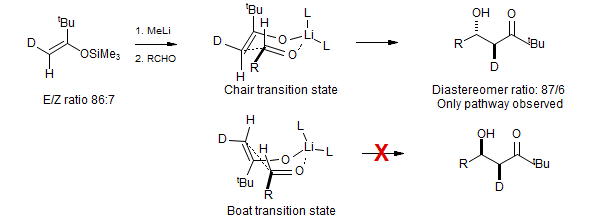
Are Aldol transition states chair-like or boat-like?
Liu, Smith, Gustin, Rousch J. Am. Chem. Soc. 2005, 127, 5770
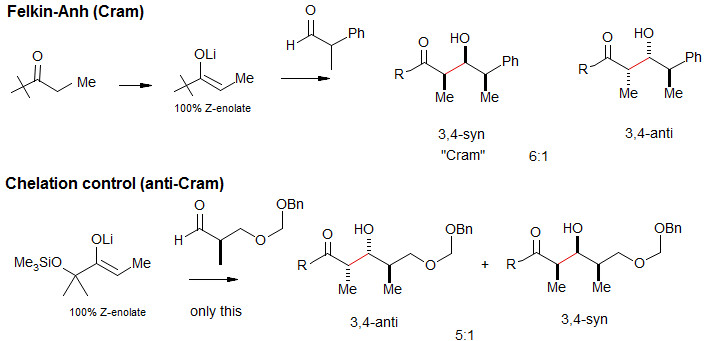
Asymmetric Induction in the Aldol Condensation: 3,4-Stereoselection
Like other nucleophilic carbonyl additions, the new stereocenters introduced during an aldol reaction are subject to stereochemical control by prexisting asymmetric centers in the aldehyde acceptor (asymmetric induction), either of the Felkin-Anh/Cram type, or the chelation control type..
Stereoselective Aldol Condensation using Boron Enolates
The next major advance in controlling aldol stereochemistry came with the discovery that boron enolates could usually be formed with high stereocontrol even with unhindered ketones, and equally important, the aldol reactions were often highly stereopecific.
D. A. Evans, E. Vogel, J. V. Nelson, J. Am. Chem. Soc, 1979, 101, 6120.
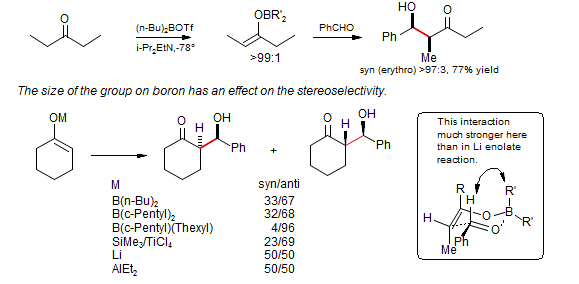
Control of Boron Enolate Stereochemistry
The Brown goup made the observation that the stereochemistry of enolization was remarkably sensitive to the nature of the base (hindered or unhindered amine), nature of the leaving group on boron (Cl, I, or OTf) or the alkyl substituents on boron (butyl 9-BBN or cyclohexyl), so that often either the Z or the E enolate can be formed with high selectivity. Brown J. Am. Chem. Soc. 1989, 111, 3441. J. Org. Chem. 1993, 58, 147.
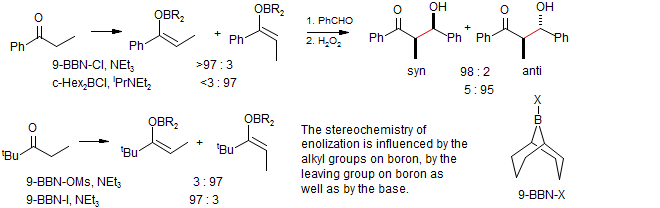
Stereoselective Enolization- Elaiolide: Patterson, Lombert, Allerton Org. Lett. 1999, 1, 19.
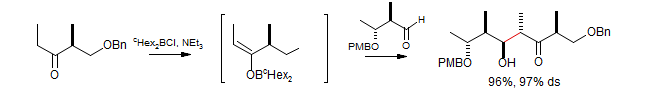
Enolization of Esters using Boron Triflates
Abiko, A.; Liu, J.-F.; Masamune, S. J. Org. Chem. 1996, 61, 2590-2591.