Chiral Enolates
Chiral Imide Enolate Alkylation
The Evans oxazolidone chiral auxiliary has been the best-studied and most successful of the many that have been used to control face-selectivity in enolate reactions. Either enantiomeric series is readily available either from natural amino acids (valinol or related amino-alcohols) or from ephedrine. These amides enolize with very high selectivity for the E isomer, and high facial selectivity ca be achieved for all of the typical reactions of enolates. A typical preparation of an Evans chiral auxiliary is shown below:
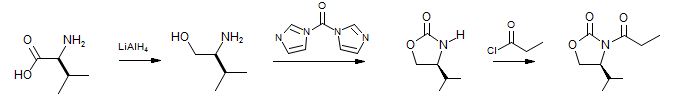
Evans, JACS, 1982, 104, 1737
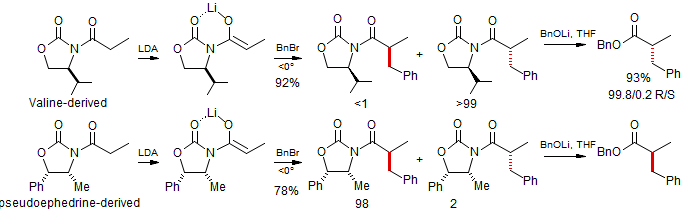
These N-acyl amide enolates show reactivity comparable to ketone enolates, so that only reactive alkylating agents work well: MeI, BnBr, AllylBr, BnOCH2Br, EtO2CCH2Br. The presence of the N-acyl group also allows relatively facile removal of the auxiliary group by acyl substitution or reduction.
Acylation
Evans, D. A.; Ennis, M. D.; Le, T. J. Am. Chem. Soc. 1984, 106, 1155
The oxazoline enolates can be acylated with the same stereochemistry as the alkylation. These keto-amides show an unusually strong resistance to enolization at the usually very acidic position between the carbonyl groups - they can be handled without epimerization.
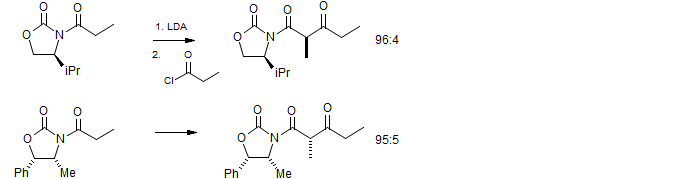
It is even possible to enolize on the ethyl side to perform additional manipulations.
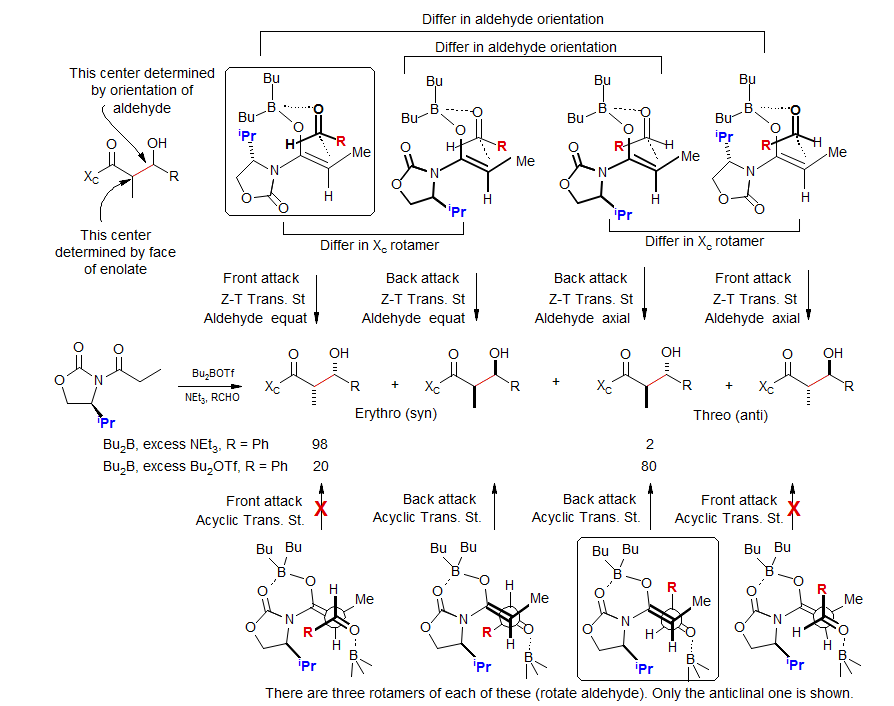
Chiral Imide Enolate Aldol Condensation - Evans System
Aldol stereochemistry is complex, giving dramatic chamges in stereoselectivity with small changes in reagent stoichiometry: Evans JACS 1981, 103, 2127; Heathcock, JOC 1990, 55, 173; Shioiri TL, 1991, 32, 7287.
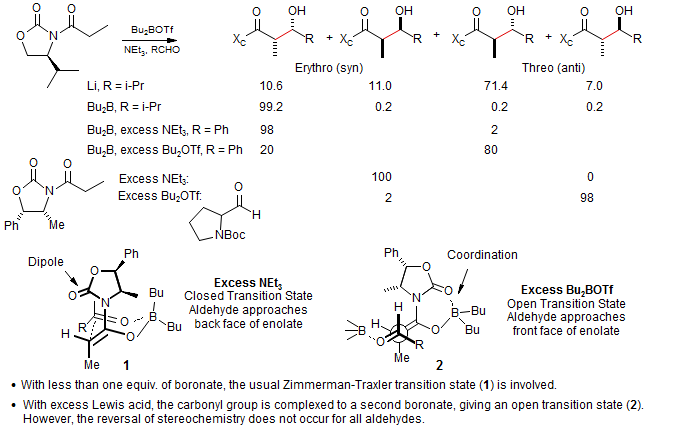
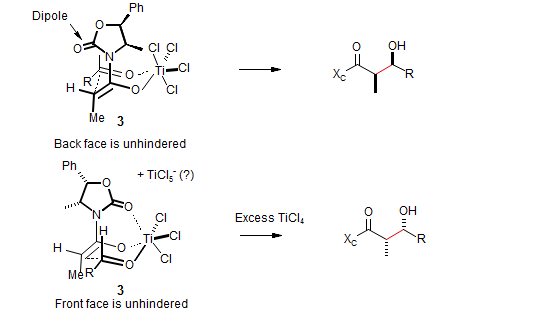
Titanium enolates normally give the same stereochemical result as do boron enolates, for the same reasons - the transition state 3 is open, analogous to 1. However, if excess TiCl4 is used, a third stereochemical mode can be found. Apparently the excess TiCl4 abstracts a chloride ion from the Ti-enolate, and the vacant site is then occupied by the oxazoline ligand, which reverses the face selectivity of the enolate (4): (Nerz-Stormes, M.; Thornton, E. R. Tetrahedron Lett. 1986, 27, 897; J. Org. Chem. 1991, 56, 2489.
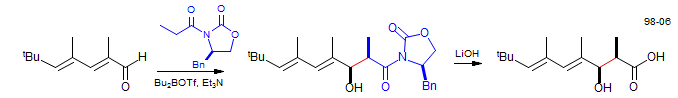
Evans Chiral Auxiliary Aldol Condensation - Synthesis of Antillatoxin Isomer
Yomakawa, Shiori J. Org. Chem. 1998, 63, 8638
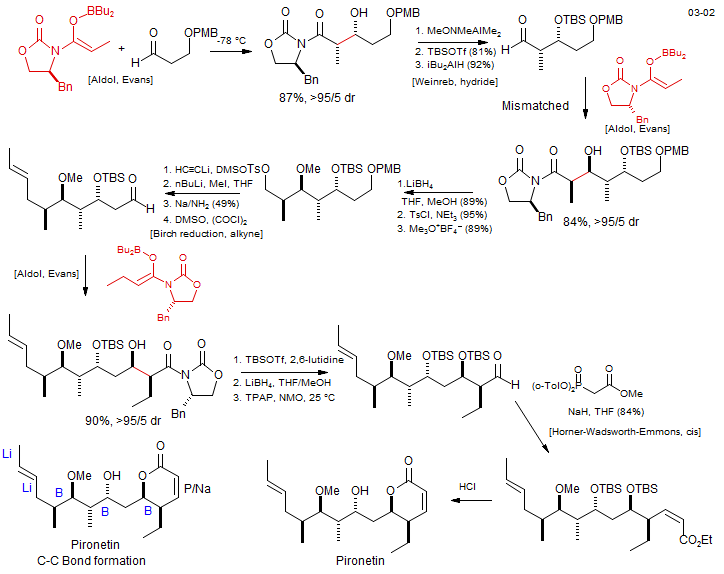
Pironetin
Dias, L. C.; Oliveira, L. G.; Sousa, M. A. Org. Lett. 2003, 5, 265
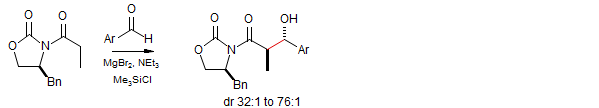
Evans Chiral Auxiliary Anti-Selective Aldol Condensation
Evans, D. A.; Tedrow, J. S.; Shaw, J. T.; Downey, C. W. J. Am. Chem. Soc. 2002, 124, 392-393
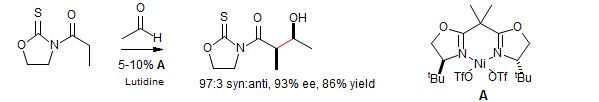
Catalytic Asymmetric Aldol
Evans, D. A.; Downey, C. W.; Hubbs, J. L. J. Am. Chem. Soc. 2003, 125, 8706-8707
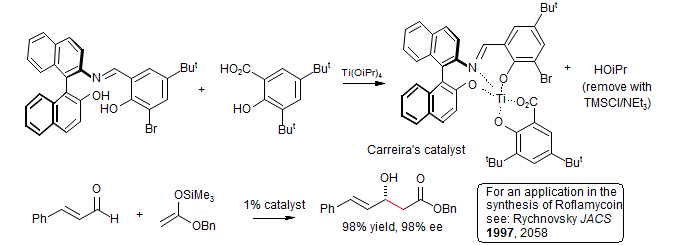
Enantioselectivity for acetate donor:
Carreira, E. M. Tetrahedron Lett. 1997, 38, 927.
Enantioselective Aldol with Methyl Ketone Enolates
Denmark, S. E.; Stavenger, R. A.; Wong, K. T. J. Org. Chem. 1998, 63, 918. Acc. Chem. Res. 2000, 33, 432
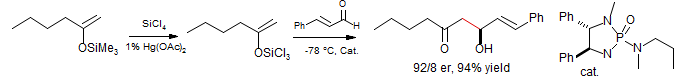
Direct Catalytic Asymmetric Aldol reaction
BINOL-lanthanide complex: Yoshikawa, Yamada, Das, Sasai, Shibasaki J. Am. Chem. Soc. 1999, 121, 4168.
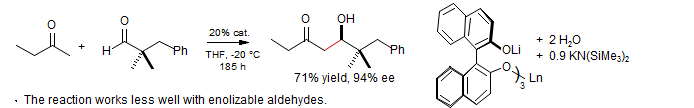
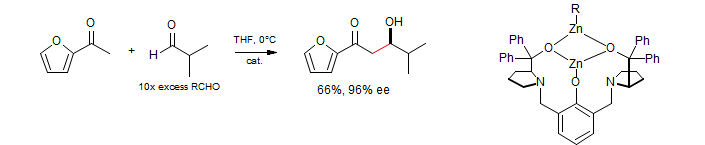
Zinc catalyzed aldol - A binuclear zinc catalyst is very effective: Trost, Ito J. Am. Chem. Soc. 2000, 122, 12003
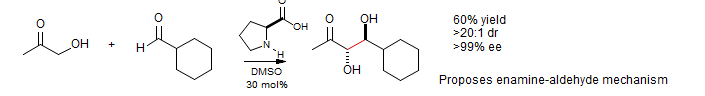
L-proline as catalyst: α-Hydroxy ketones seem to work especially well in the direct crossed aldol: List J. Am. Chem. Soc. 2000, 122, 7386
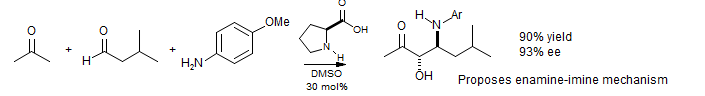
L-proline catalyzed crossed Mannich condensation: List J. Am. Chem. Soc. 2000, 122, 9336
Enantioselective α-Fluorination of Carbonyl Compounds: Organocatalysis or Metal Catalysis?
Pihko, P. M. Angew. Chem. Int . Ed. 2006, 45, 544-7.