Conformational Energies (A-Values)
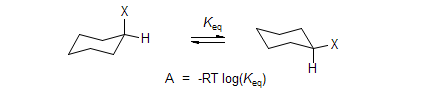
| Group | A-Value (kcal/mol) | Group | A-Value (kcal/mol) |
|---|---|---|---|
| Me | 1.70 | Et | 1.75 |
| CF3 | 2.1 | CH=CH2 | 1.35, 1.688 |
| C≡N | 0.17, 0.242a, 0.219 | C≡C-H | 0.412a, 0.529 |
| CH2CMe3 | 2.0 | CH2OTs | 1.75 |
| i-Pr | 2.15 | c-Hexyl | 2.15 |
| t-Bu | >4.5 | Ph | 3.0 |
| CO2-* | 1.92 | CO2H* | 1.35 |
| CO2Me | 1.27, 1.312a | CO2iPr | 1.20 |
| C(=O)H | 0.8 | C(=O)Cl | 1.25 |
| C(=O)Me | 1.17 | - | - |
| D | 0.0081 | T | 0.011 |
| F | 0.15, 0.282a, 0.369 | Cl | 0.43, 0.532a, 0.519 |
| Br | 0.38, 0.482a9 | I | 0.43, 0.472a, 0.499 |
| O-Me | 0.60, 0.759 | O-CD3 | 0.56, 0.552a |
| O-Et | 0.9 | O-H* | 0.87, 1.09 |
| O-Ac | 0.6, 0.712a, 0.799 | O-C(=O)CF3 | 0.68, 0.589 |
| O-CHO | 0.27, 0.592a, 0.629 | O-Ts | 0.50, 0.522a, 0.489 |
| O-Ms | 0.562a | O-NO2 | 0.59 |
| O-SiMe3 | 0.749, 1.3110, 0.8011 | O-SiiPr3 | 0.519, 0.9410 |
| O-SiMe2tBu | 1.0610 | O-SiPh2tBu | 0.5610 |
| S-H | 1.172a, 1.229 | S- | 1.3 |
| S-C≡N | 1.232a | S-Me | 0.7, 1.072a, 1.12a |
| S-Ph | 0.8 | - | - |
| S(O)Ph | 1.9 | S(O)Me | 1.2 |
| S(O)2Ph | 2.5 | S(O)2Me | 2.5 |
| Se-Ph | 1.13 | Te-Ph | 0.9 |
| Group | A-Value (kcal/mol) | Group | A-Value (kcal/mol) |
| NC | 0.212a, 0.199 | N=C=O | 0.512a |
| N=C=S | 0.282a, 0.219 | N=C=NR2b | 1.0 |
| NH2* | 1.2-1.7, 1.239 | NHMe* | 1.0 |
| NMe2 | 2.1 | NH3+ | 1.9 |
| NHSiMe3 | 1.2111 | ||
| N3 | 0.629 | NO2 | 1.052a, 1.139 |
| PH2 | 1.64,12 | PMe2 | 1.54,12 |
| PCl2 | 1.94,12 | P(OMe)2 | 1.54,12 |
| PMe3+ | >3.04,12 | P(S)Me2 | >3.04,12 |
| P(O)Ph2 | 2.65 | - | - |
| SiCl3 | 0.612a | SiMe3 | 2.56 |
| GeMe3 | 2.17 | GePh3 | 2.97 |
| SnMe3 | 1.17 | SnPh3 | 1.47 |
| PbMe3 | 0.77 | - | - |
| HgBr | 0.0 | HgCl | -0.3 |
| HgOAc | 0.02a | - | - |
| MgBr (Et2O) | 0.782c | Mg-C6H11 | 0.532c |
* Solvent dependent.
General References
- Hirsch, Topics in Stereochemistry, 1967, 3, 199.
- Jensen, F. R.; Bushweller, C. H. Adv. Alicyclic Chem 1971, 3, 139.
- Eliel, E. L.; Wilen, S. H.; Stereochemistry of Organic Compounds, Wiley, 1993, p. 696.
- Eliel, E. L.; Wilen, S. H.; Doyle, M. P. Basic Organic Stereochemistry, Wiley, 2001, p. 443.
Specific References to the Values above
- Tetrahedron Lett. 1989, 30, 1059.
- By direct NMR observation of 1H signals of axial and equatorial conformers at low-temperature: (a) Jensen F. R.; Bushweller, C. H.; Beck, B. H. J. Am. Chem. Soc. 1969, 91,344. (b) Bushweller, C. H.; O'Neil, J. W. J. Org. Chem. 1970, 35,276. (c) In ether: Jensen F. R.; Nakamaye, K. L J. Am. Chem. Soc. 1968, 90,3248.
- Duddeck, H.; Wagner, P.; Gegner, S. Tetrahedron Lett. 1985, 26, 1205.
- Gordon, M. D.; Quin, L. D. J. Am. Chem. Soc. 1976, 98, 15; J. Org. Chem. 1976, 41,1690.
- Juaristi, E.; Lopez-Nunez, N. A.; Glass, R. S.; Petsom, A.; Hutchins, R. O.; Stercho, J. P. J. Org. Chem. 1986, 51, 1357.
- Kitching, W.; Olszowy, H. A.; Drew, G. M.; Adcock; W. J. Org. Chem. 1982, 47, 5153.
- Kitching, W.; Olszowy, H. A.; Harvey, K. J. Org. Chem. 1982, 47, 1893.
- Eliel, E. L. J. Org. Chem. 1981, 46, 1959.
- Direct observation of 13C NMR signals of axial and equatorial conformers in toluene-d8: Schneider, H. J.; Hoppen, V. J. Org. Chem. 1978, 43, 3866.
- (a) The A-values for OSiR3 are unusually sensitive to solvent. These values in CD2Cl2 from low-temperature 13C NMR: Eliel, E. L.; Satici, H. J. Org. Chem. 1994, 59, 688. (b) Marzabadi, C. H.; Anderson, J. E.; Gonzalez-Outeirino, J.; Gaffney, P. R. J; White, C. G. H; Tocher, D. A.; Todaro, L. J. J. Am. Chem. Soc. 2003, 125,15163.
- Hardy, J. P.; Cumming, W. D. J. Am. Chem. Soc. 1971, 93, 928.
- Using the "counterpoise" method: low-temperature NMR of 4-methylcyclohexyl derivatives.