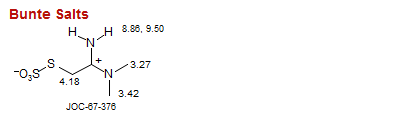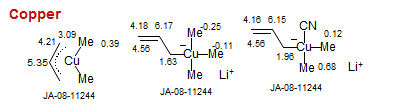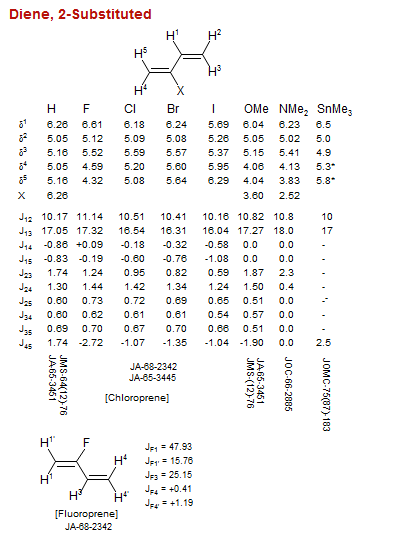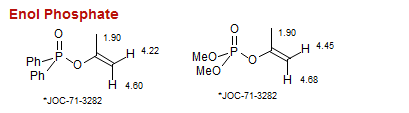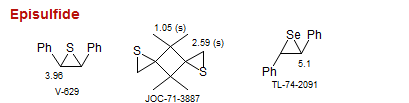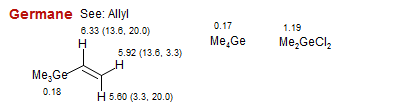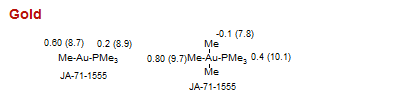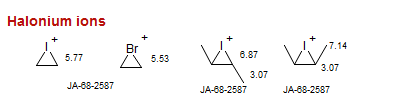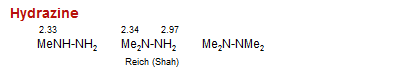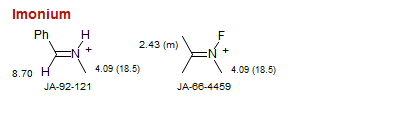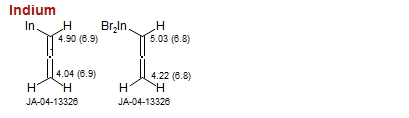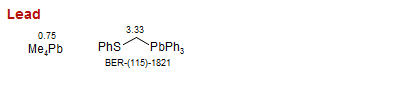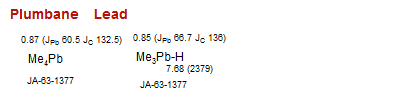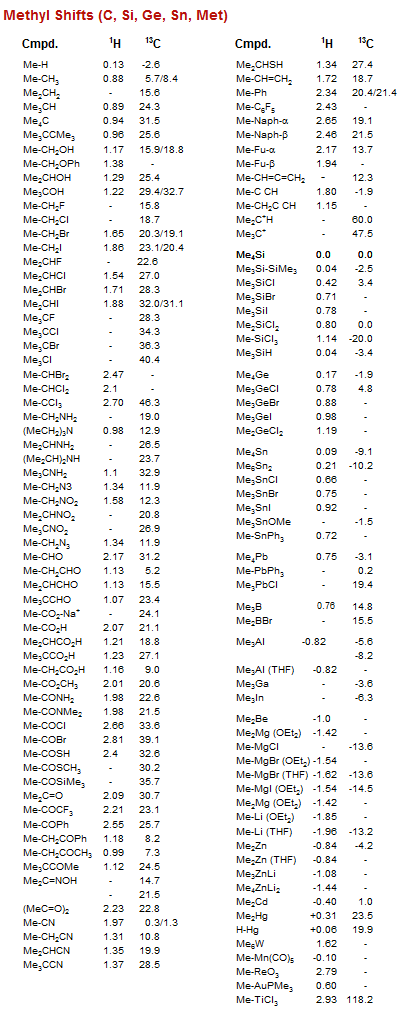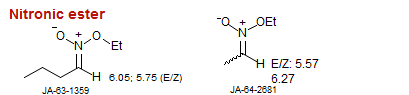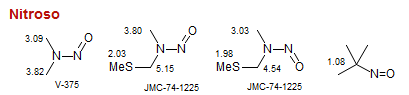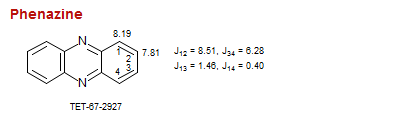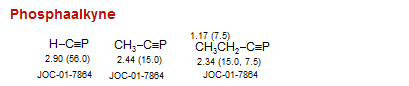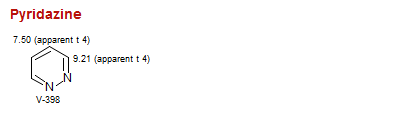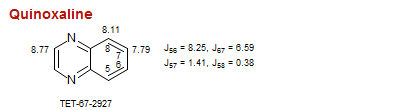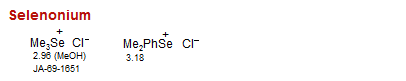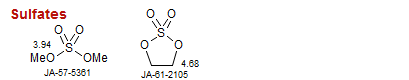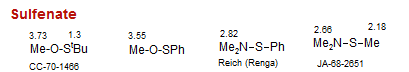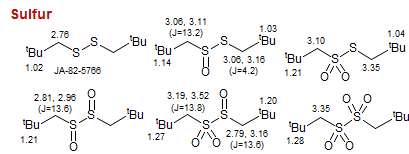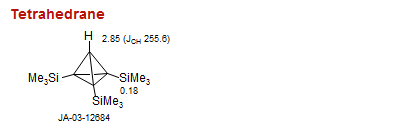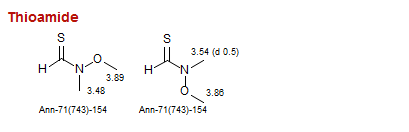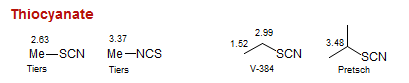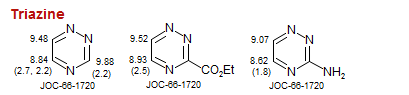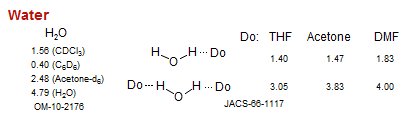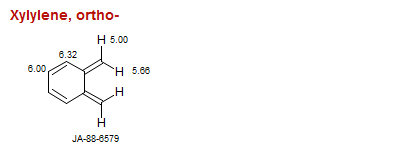Explanation
For tables of data see Acetylene: Alkene; Allyl; Allene; Benzene; Benzyl; Cyclopropane; Cyclohexane; Diene; Naphthalene; Neopentyl. Methyl chemical shifts are listed in several Methyl tables. Heterocycles are listed under Cycloalkanes, Heterocyles: Bicyclo[x.y.z]; as well as under specific names (e.g., Pyridine; Furan; etc).
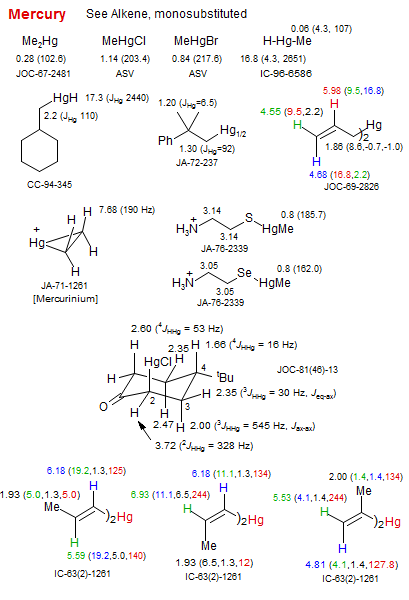
Chemical shifts marked with an asterisk (2.42*, 2.47*) have not been unambiguously assigned.
A references such as JOC-89-4436 is a coded form of J. Org. Chem. 1989, 54, 4436. An asterisk next to a reference (*JOC-78-233) indicates that the paper has 1H data for numerous other related compounds.
Numbers in prentheses next to a chemical shift are coupling constants (usually JH-H but sometimes JH-C or JH-X if the molecule contains a spin 1/2 nucleus X (e.g. P, F, Sn, Se)
Acknowledgments
This website is made possible by a generous allocation of server space and bandwidth from, previously, the Department of Chemistry at the University of Wisconsin, and, now, the ACS Division of Organic Chemistry. The National Science Foundation is acknowledged for financial support to Professor Hans Reich who created the database of NMR. Dr. Khoi Van (Assistant Webmaster of the ACS Division of Organic Chemistry) is acknowledged for his extensive volunteer efforts in converting Professor Reich's database out of frames into its current website (responsive form) in June 2020.
Reference Abbreviations
A references such as JOC-89-4436 is a coded form of J. Org. Chem. 1989, 54, 4436 (89 is the year). This could also be coded as JOC-(54)-4436 (volume in parentheses).
ACIE Angew. Chem. Int. Ed. Engl. ACS Acta Chimica Scandinavica AMR Advances in Magentic Resonance Ann Justus Liebig's Annalen. ASV Aldrich Spectral Viewer BCSJ Bull. Chem. Soc. Jap. BER Chem. Ber. CC J. Chem. Soc., Chem. Commun, CEJ Chemistry - A European Journal CJC Can. J. Chem. EJC European Journal of Chemistry EJIC European Journal of Inorganic Chemistry Friebolin Frebolin, H. "Basic One- and Two-Dimensional NMR Spectroscopy,"" 2nd Ed. VCH Publishers, New York, 1993. Heterocycles HET Heterocycles Hesse Hesse, M.; Meier, H.; Zech, B. "Spektroscopische Methoden in der Organischen Chemie", Thieme, 1987. IC Inorg. Chem. JA J. Am. Chem, Soc. JCSP2 J. Chem. Soc. Perk. II JMR J. Magn. Res. JMS J. Mol. Spectr. JOC J. Org. Chem. JOMC J. Organomet. Chem. JPC J. Phys. Chem. MGMC Main Group Metal Chem MRC Magnetic Resonance in Chemistry NMRShiftDB https://nmrshiftdb.nmr.uni-koeln.de/ OL Organic Letters OMR Org. Magn. Res. ORGMET Organometallics Pretsch Tables of Spectral Data for Structure Determination of Organic Compounds Reich Results from the author's research RTC Rec. Trav. Chim. SC Synthetic Communications SDBS Web Spectral Database for Organic Compounds S Sadler Spectra Collection SL Synlett TET Tetrahedron TL Tetrahedron Lett V Varian Spectra Catalog ZOK Zh. Org. Khim (Journal of Organic Chemistry of the USSR) -Shift Table 1: δ -10 to 2

-Shift Table 2: δ 2 to 6

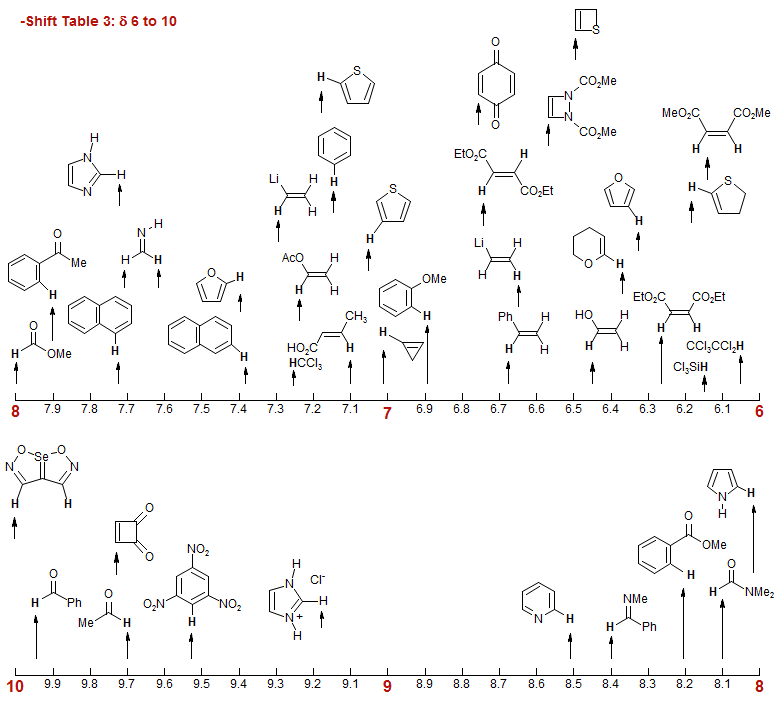
-Shift Table 3: δ 6 to 10

-Shift Table 4: δ 10 to 20

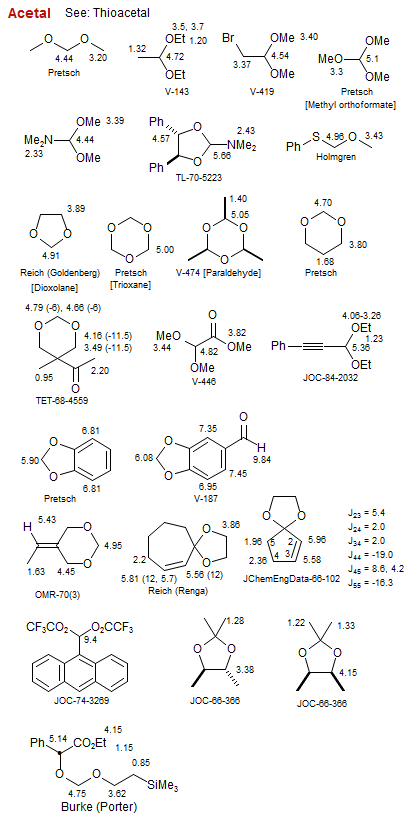
Acetal

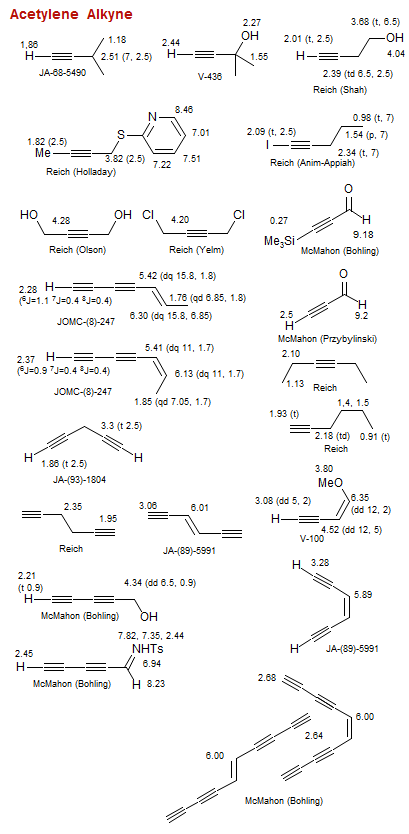
Acetylene

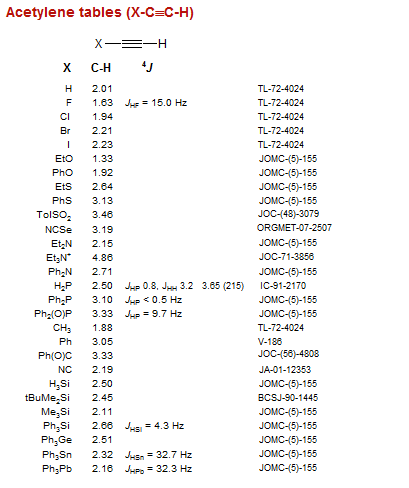
Acetylene tables (X-C≡C-H)

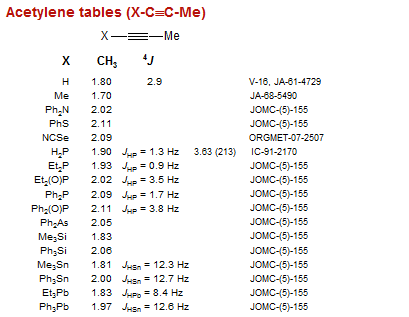
Acetylene tables (X-C≡C-Me)

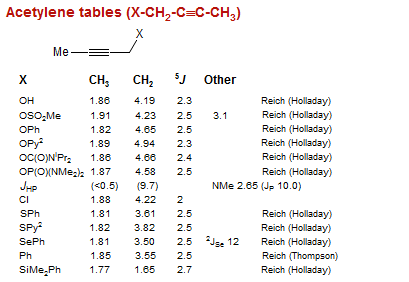
Acetylene tables (X-CH2-C≡C-CH3)

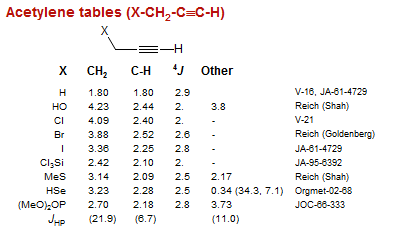
Acetylene tables (X-CH2-C≡C-H)

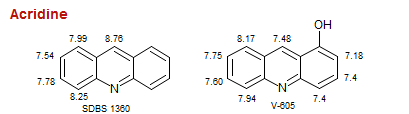
Acridine

Adamantane

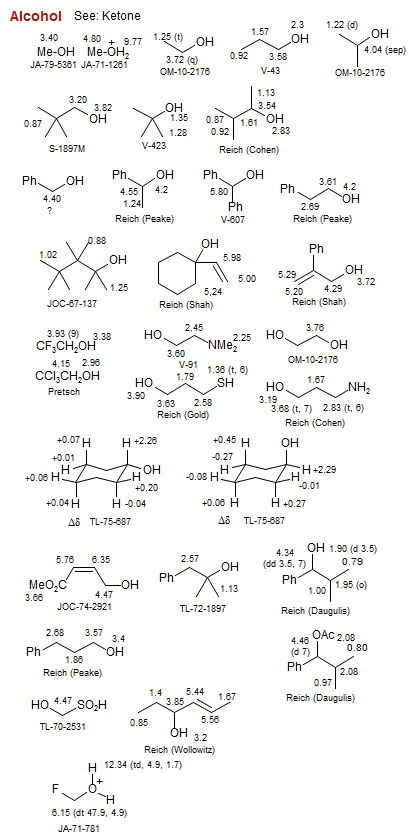
Alcohol

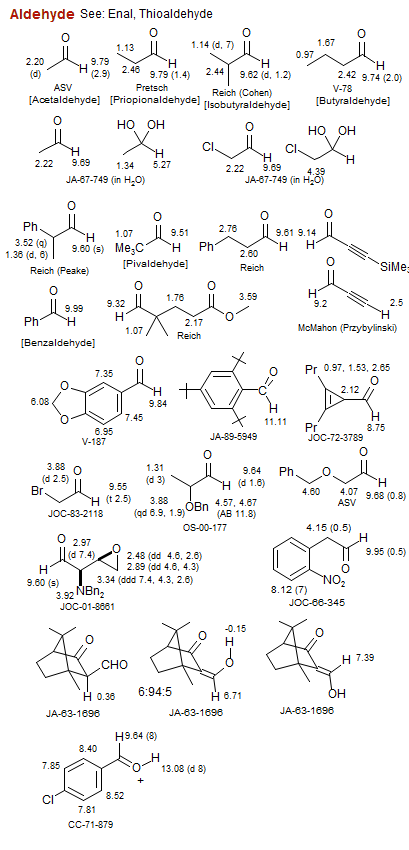
Aldehyde

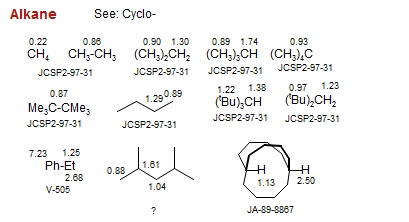
Alkane

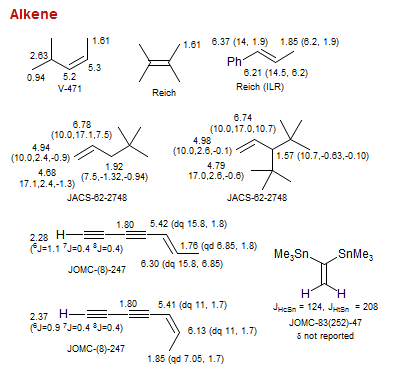
Alkene

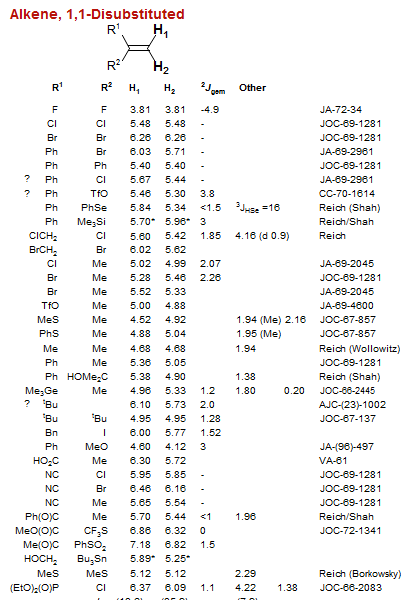
Alkene, 1,1-Disubstituted

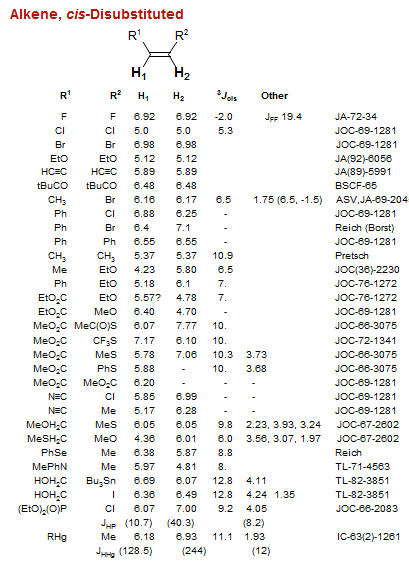
Alkene, cis-Disubstituted

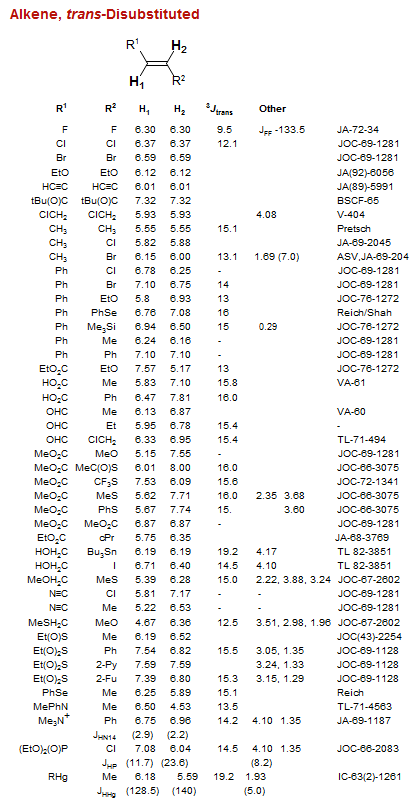
Alkene, trans-Disubstituted

Alkene, Monosubstituted (C-Li)

Alkene, Monosubstituted (F-N)

Alkene, Substituent Effects

Alkene, Vinyl Halide

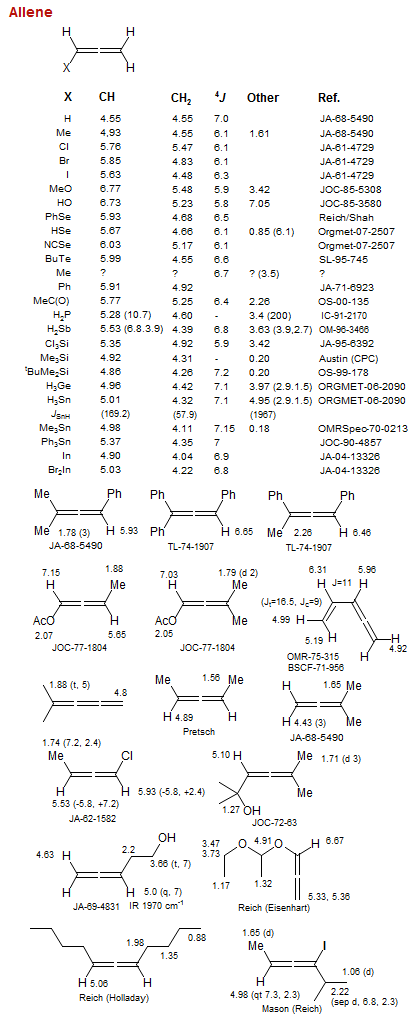
Allene

Allene Oxide

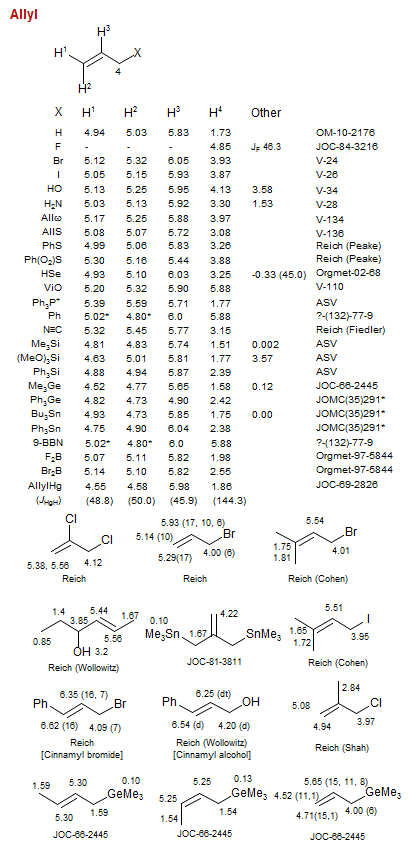
Allyl

Aluminum

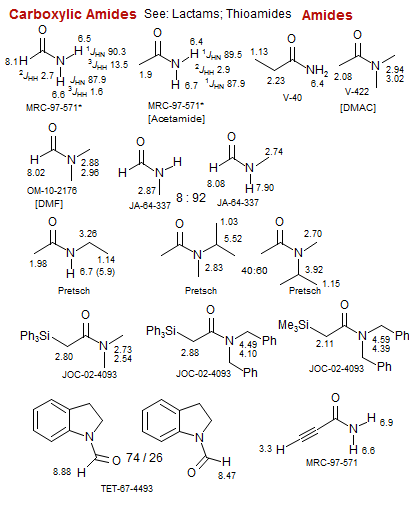
Amides

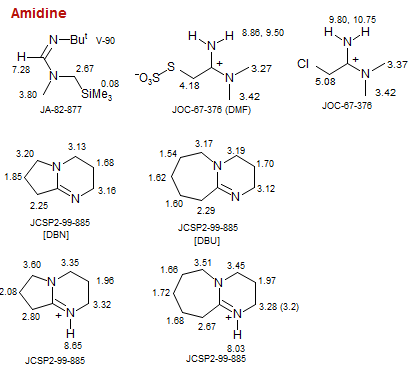
Amidine

Amidine

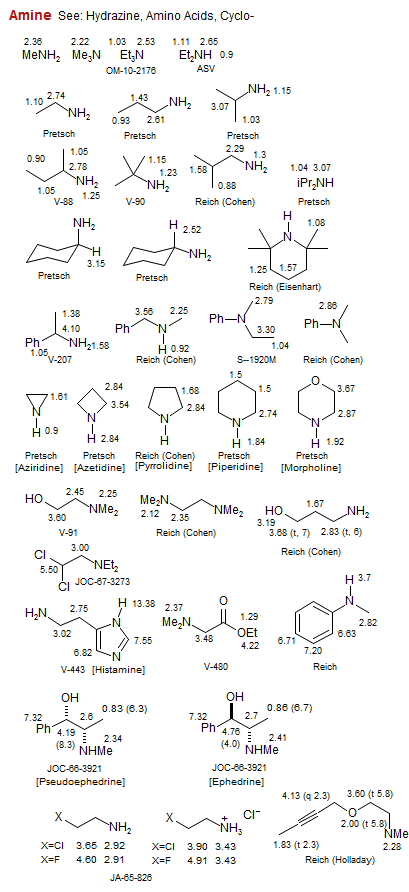
Amine

Amine, N-Hydroxy

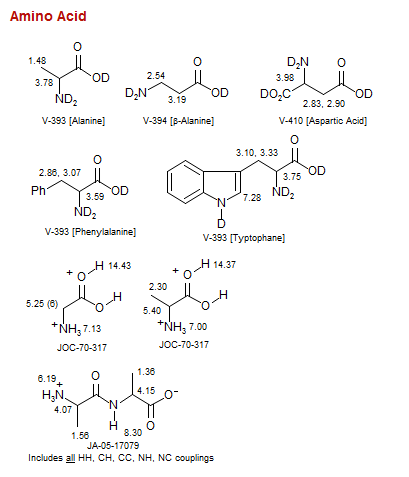
Amino Acid

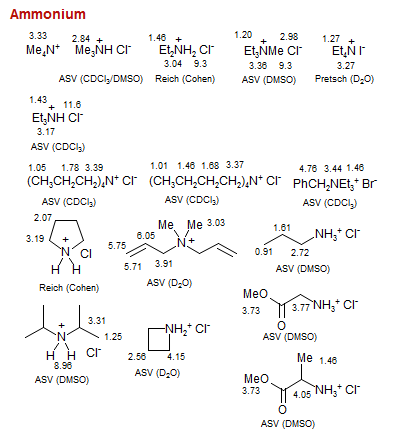
Ammonium

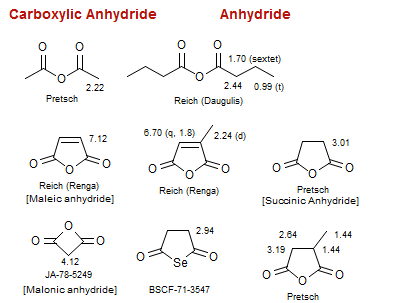
Anhydride

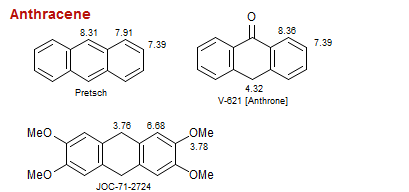
Anthracene

Antimony

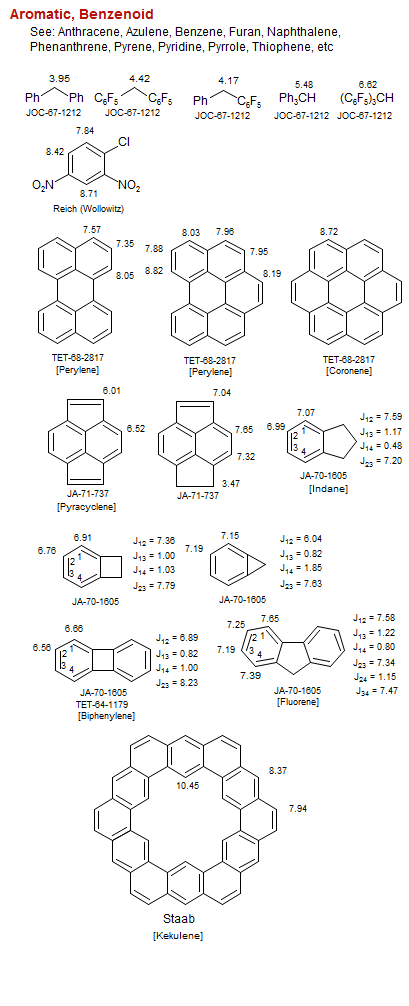
Aromatic, Benzenoid

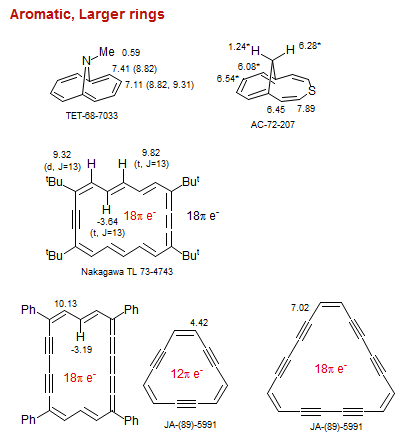
Aromatic, Larger rings

Arsenic

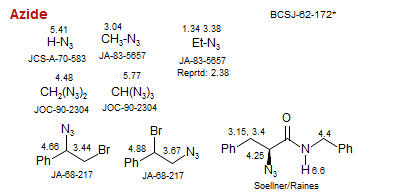
Azide

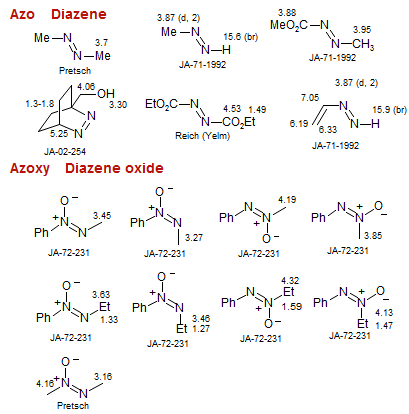
Azo

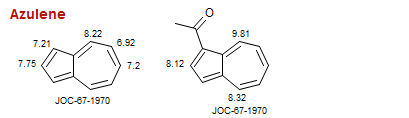
Azulene

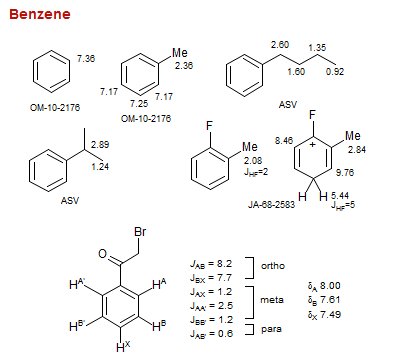
Benzene

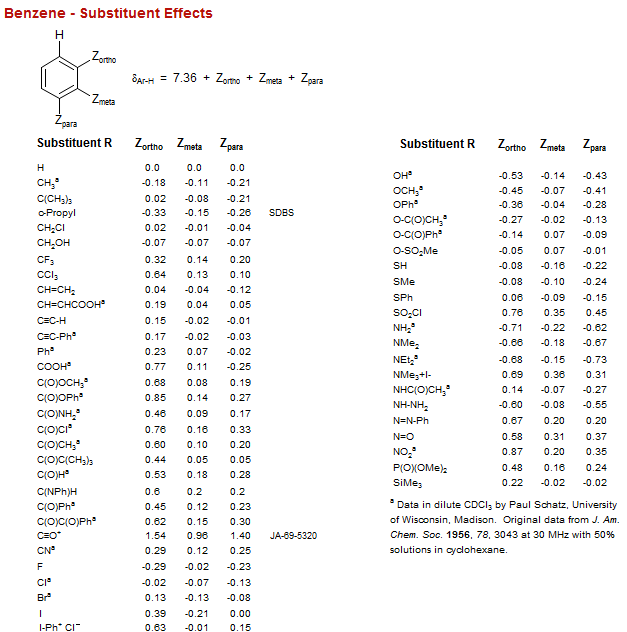
Benzene - Substituent Effects

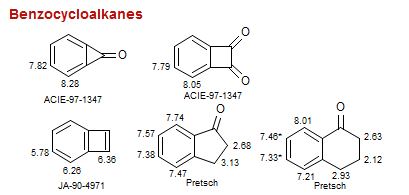
Benzocycloalkanes

Benzofuran

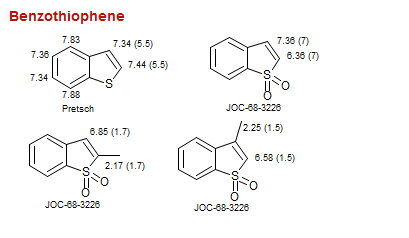
Benzothiophene

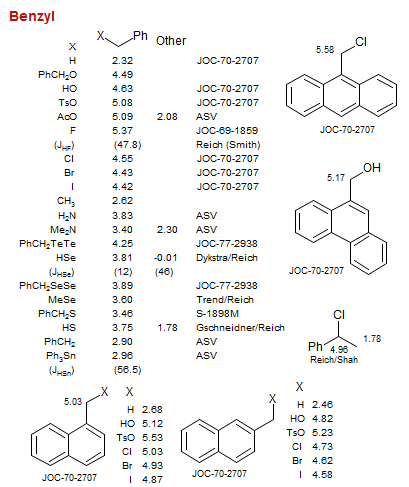
Benzyl

Beryllium

Bicyclo[1.1.0]
![Bicyclo[1.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAACpElEQVQozw2RS08TUQBGbwHlUR6FAkLlVShQCI/ISwzGGGNcuDC6hJCQGMUCHSjlUTrtzNw7M3fuPO+dDoVSMNGFceXKKCauXIsLlQ0Lty5cGX8B9g98J9854Ae3sviTi82fr68tfE9yj862k0++prbvfEsmB/5uc5WXAADqmM2mbbZhogVtx7haPD6szheOGgvFYt1R8ajy5KToO/t1CdSsVG8Tp5carC/vHQf//P4HkKwDcB6PTZ1xK7NfEty9Dzub99/vpR6cZvnJzzzf/QnLfvekUMUYbdUI6YBQ6oGS0GGaehfLeT1ePt+e299vYDlWsbOVBkpGrBMzUkTKwEFBgCFd1ysEEZaDi9XngY8ba6E3ycRgIZ2aKkrizEsFRV9rOHRkm42lkWbmul1E18MIwQgUs/2GQaLMZaPewUG/d3DY4ua8msTaxpXlp886Y8uxifgaN7m7m4pgjBswJn5wur0JHkP5akIQrqUlaUhUlRGok06N2k2Oy+rdXK7ZdmininEEQjiUzfCjMpLGNILHHdftpfv5Jsdxq9Nbe7WL84tdC/MLE0tLS1OJRGKEENJumFYQvDhkwEJCDZZhSMPKmKbhG7qhh0tXA5SySsZYnWGabZpGSiC1uwTplsRMH5JhxKJOiBWO/dRmVTArtHLx+PDq6uqteJyb5nl+2DCMdsehASBmdwGRBT+CYlhV5RlZkWdVTevLIlT3Cq8DqkEfklA1wWor0bVeRVWGIZIGM6JwHcqSX2SH5aXgZaaGA6qMeqEkTUOEbkJZiZYMBN2cWwWSSQ4oEt8oCpmBkvPbEME5WVWjvCg2Pixc+HKU+BzNLPeo3UCZHbaoNU4sYwCbegvESkXx7TsflpUyz7VqXWb1UWrPOZTeNW06ZDEvEOdV8B+ZHyd1CLSsFgAAAABJRU5ErkJggg==)
Bicyclo[1.1.1]
![Bicyclo[1.1.1]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABIAAAAZCAYAAAA8CX6UAAAACXBIWXMAAAPoAAAD6AG1e1JrAAAFfUlEQVQ4y12Uy28b1xXGryVLqizrRVEiRYoUKYmULNNxotZBkT8mmxZoFkGyCBo3iWWJM3Pfr3lyZkiKlGS1iJ22QWOk6KIt0GYRIEFQoIsii2zyV2QToD102oV6gbMY3Jnf/e53vjPo29dff/WbX7zx2tdvvvnTf73zzv2/v/f+7hNCGh6Fct1a96S7Qjwz8/DhuzOe213yXFwQTC24bjRtbIIo9ZGSEqEvf/bzvb++9fa9Tx8+PPrk+PFLzzy3mRKy5bhuw+k6O1BlgvnC8fHxavf0tOJ5uCalWtY6nUmGzxATOQp8HyH5waOaPDk90IQ8sEIcBdbsSCE3sefVsYtbBJO65KJAMK5DtSjjLa1NIQyzuV+9/QYaffw5Uowi5IACF+N9yvl9rmRHW9MQQtQYYzuEkDaltKo4W5ectTlj97mU+1zr5eHZ2fQnf/4KcSGRH8cIcexVOKV1KeWe0rphrNlQSq9ppcoArAopClaKolGqCV7cE0q1hNRla4PbSppZLP0bKh0h5Gs+xxlZAwWbjPKS1v48dr0lRmkJFJUxo4sMe0VOyTYoOiBwKCG0Tilf50wschNN9Z48Q+jdXz6aOjl+XHAdp+o5XplScevRo8er3ZPTUve0u+E47goYXnEdd5t4pDK5KhxyyBg/5FyUhI2nw8EFXM3zJgomRrY9jLdBxXL3xNl0u04DIFtgdhlj0oD9XdhbpwRDkV0AtsH4da6jqSgbAMhx5inGJQrSMSUlSjzIDYaWe00AVycg+GgLrr7LBNvggq1xzkpQm0yIRSL8KSEtQkaKFa0kGMubTPAavLChpFqVghel0kVt9Lo1uqaU2pXQACrUbcnJTcroHOH85qknbsAeQkqrJYAUOKc1xvmk9SVjggVrxKIN7BqAykqJBnSvBZ3dYtq/lQ36KAp9hKVBUgco9AMAKVUA6RVCcBvkd+C5FYRRKY6CYhQFVWvNnoR8wQH3lJIHfhBUgii6lY4vpv/9RYb6oUTfI1iBVcugqMoZPRSCHRmjOvBiI4rDahgFdeubFoBelko80FodhVG4F/d6q9loPHuR+WicavTWX75DKDQcgjXxhr4MoNeUlj+GU1thFFXCMNjwfVMFUBtA9wB0CKBGL0tX8vF49hIgF6n6H4jdtlpWwNyO4PwnAtJrrL8VRPFtSPl8FNhJM3bgyodg9k4QhoXhaDiT9fOpYc9HZ3n4A0gyd04Jvgr52IGsdCAzd7gQW8qYJWPlDKNkAQa4ij2y7blekRAxncBsPfn9U3SeSDROxH8VhXYJTN0AM5sA2p/A4Hr7fhhshnG4IBhbfRFAwg4wpiUu1KzlChlj0TANUZ7YH0BJEi/ESbQulNgjFHc84sJgija0vhzF0S2G8TLAa6AUAkpWMOPTlBGECUa9JELPnj5FL1aeJfNZHGwOjD7MrDzKrXmll+V3++OrWv7R7xZNHL1QJYQsUiHnoaaUFvD74OjaCrWYyq1avFCiPjT27thPDtL+ZSMdfViU/bNVV9sF5pF5mKtZj9FppsyNAMIotbwOgt/I9Hli5j9UfGOg1N657LXS6Hw3z35dkVFSZ8bfsNpf7qX5zb998RmySYp6eY7M/4OoUSh4bmaJOl07dZwm/0A1fTdupCKF+dL70L12HMfNfJAXRxfj+bSf3/jjx1fo8nJ0HfQ1TO7VH8yMZidFTLoN7kBWdNJKbe9u4NsHEMBXe2nvKB/0dwZnw5V8NJx6/vy3aHw5vg76h4Z0Xsm5UDhlo/muVsGdIMkP4qjXicKgAx3tpFl6Jx/26/3hYHkCevrRbxCouw76ZxyiPyl9c8xZIY/CbdtL7ug8a5s4bIWB3wRQI0l7tTTP1uIs+1F+Pkb9qyuUjq6D/gNGBe07+OCcpgAAAABJRU5ErkJggg==)
Bicyclo[2.1.0]
![Bicyclo[2.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAKCAYAAABBq/VWAAAACXBIWXMAAAPoAAAD6AG1e1JrAAADe0lEQVQozxVRyW5bVRg+SYA2CRlbx4mHOG4S20ljpzZ12bCgOySQeAAeILN97Q7xPJ15uOfce22H1LSlg6BC7MoCsWAFUhcsIOUFeAlWLMLtkf7F0T98E3h7cvC5X5+9LR5/cVE4vPvXydGnF9bx7p9WMXrRrk2Cy0sgOtUPKEWzhLNZpfUMZWxaCr5AuZg1rns1/1UBvHuX/uyX+y+B0XysP/TGpfHGf/3nPwB+Py3f+e1h+fabB+XcH9ZJ6s298vYv9UrqdasZ+QGhKX7uTDDYnOGMRCnjq0LZUQjRMuf0BuU8YlwTRIx9KASbtDV//8zWV2wh5jlX84jLyaYcjIHvu63YC9hdf9Fux1/WaivfNRrLjzGNP1Jq1Ui16HlyGnUbAcbIDqEsw6Xc8UE2BKdZJkTCdZ01LlSAYLKEMFok3d6cYiKkpAkLqa9554/eA0qr68LzgnWEV6xaPfCgVl8hTMZsY+JSmZDr9zHshRjFGUzoLf9wutuDCV9JjgqWdF0Tk7ZZ7Ha6wdPKaajy8DRMIIob5dzQthPRenAN2JXm1YF0ZtuNVrhklTYLxyfb98v3UwTCBCNszdV2CEMYoRSnMaVZoWQGYZzknGQxo5uOY4JKDae6rU6gWLQ2D/YPtgsnhXSn1U76+5vaeKvg2ZMn4MwbTPlMQpZl3dzb28seHR7l65Xqbq+D4p5tByiEYR8kQznLKm37ikjS/+cgRklpyyUlzCTDdLFklTf29/fTR4eHH9ertTzqkbRS7joYfX0OBq43I4UMVk4rW8VCIXevVL7barQ+gZBsOkYHUK+9TAjM+MyzXIldRHDKt+8jREhKaRmUXE33HW++UW/Gy6XSllUs5WvVWq7XxVu2cmLgrN8HrtbTRps5ivBao1pPtOvNfK8Ds4ypiLbVYqtRWcKoncaM5KRWuxDjbUxgHhK842ca1tqZHA2HV7S0lyjGUQTRNoI4SYiK2NJbAKMzCEbfuGNPn43G+46eN0qFbS5SvrKbUuk1Zatws14JwV4z49tzWyiR6fl5EYIyhLGE0/eW1ej5RLnngKHrzLtGR43RSaVN1NbegvbzAt8+9sDrn16By3//BgPPmR64JqAEjTNONwjnMaVkuNWoriLYuuXbdMc2tq8ErROMEj5IzB0MrpunP07I4XPfdj1nK74mJHtHIE6FmpP9n8f+Bw6vrD2oUtuUAAAAAElFTkSuQmCC)
Bicyclo[2.1.1]
![Bicyclo[2.1.1]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAJCAYAAADHP4f4AAAACXBIWXMAAAPoAAAD6AG1e1JrAAADI0lEQVQoz03Tz28bRRQH8OfYTe2G/FSSUuxEdlRD7KRpiITgwA8JTqicuZULQsVJYzvOxrveXzO7453d+bXr2KRpBY2ClApxR70UCfVYCZSm6l/ABfFXlC3iwEhzmvd9n/cOAy92t2+9aDY+v2jd/eKiuf3peav54fP91tq5pl19ia38n9iA58gCRkO4/+gMHngBKBpnmB9lEOHw8w8/wSDqQ6ezC829JjRaLdBsAm2LwJ7hwr/nYrdRu2g2Vp912jefHnQ2fzWMm09NffkPw5x8pe+MPfYQ/MUZiMEAfvnxEeBKHSSV+ZgdXomTe4Xj788yMfXHNK2VbXfa2e12eyxFMt92Meg2AjMaApw3vim8vHun8LjbnT3tGcVjF62MPK88pOzNhLIpEsaXWMgBuU6WsjAfc1YQEZ8JCV0M+mGJBuE8wd6srmuzHa0zv9Pem9jRzJyBPIDrH4HDvwP4++uv4NUnm9ClYsIlwTUvoOU+DStMqArnsshlPOViNI5cd9pxnJJp9t6yLWsFu27V98hqEASlgPhF2+pVurpe3e1oC3tds/DOZ19Cz0FgsxGApxvZPsZTHkKltNH1NFymhCwLLsqcq9J/SMF13QWM8dvpXU2xNce21tPaTRoEtYjSNYTdG0bPeLe9r1W0g4PFtH7CdP3L8b3TDOxrxpjv4VkXoRXPw3VCyAqPWEnJuCSkuirieBJhXEgbz3gYl4lP6p7nb2GENzyEayGlVcF5nVJaSwdY65nmUrrtou04KULG/49MY4yW+8TfCPp0I0VWWSSXmeAzRA4uG04/Y/R6uUSJqUEyKCqp0ne+zmi0mW5cl0LUIsaW0vyS69jzlmnOWZZ95TUyenCWgcPIAx6QS8T35lhE19PwB5KL91ikqhFncz6P88TRYXQo4fThUe746Gg6iZOKkvJGCmwJKauMy2tSqclEycmj4Wh8kAyzsVJw6/ZtQOIIYNQnKRTlwqC/oATbkkJ+HKt4K2JxkUb9vB0mOTP9AwT7cP/EhWEixwVnxVi9BsT7MlYVP1Jv7GsJqJhnnvz2O5ycPATdMACRADx1DP8ASKlujO7kl/wAAAAASUVORK5CYII=)
Bicyclo[2.2.1]
![Bicyclo[2.2.1]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAAA0AAAAZCAYAAADqrKTxAAAACXBIWXMAAAPoAAAD6AG1e1JrAAAETUlEQVQ4yxVUWW4bRxBty4pk01oskqIoDjmURMqSScdwZCBA4B9fIjdIkMAGYsHWxmWW3mbp2Wd6hqQsWwb8YQcBggDJR4DkBEE+cgXfIMgJUvwoNGZQVe/Ve9WNfmXfdH6xnt3/6LzqXTnGQeyK/chPepEf9ZIo2UmSuJpPilIxKZaK2WyhkAlCH8RJ870/3n8b0n7uOz2Lsj7F7BGn/GEYhN00TeuyyNdkLpflZLqQplA0yZOqlMlOLtNeEAQPTQMfjQbjLw3dOPJ9vydlpso8L2dS3kqLyUKcSSgq0rLMs0aeZ3uQdEgweaxp+lfYxEee53UAoSqLye00zRa+Hr1GL623CBVFVkqzeCNLk7rveW3bsnucW1+4jtsPwlBNk6Qax+lqmqRLyezNwtufPyJkGvoyY2zVse0NzxU1T4hdz/f7QLXrB4EC/+uO7SjQZEME6fLvf/6GECHmOudMsW1bhWjanO9Ytn3gum7bdtz5955tOQ9cR7SEn95hREcIY71CKFEpYyogNiglLUJph3LeYJxvcUY7nPPPOeM7hDp3njx9ipDFyRrjtMosVoHzLqV408SmYmJc1wy9grGhEIzboOb22fnwzrfffb+ITk5Oaufn51vj0bjKGN0ApJpu6E3NMBpjbVwf65qi6ePmYHihnF2cV1+dnd1Fz5893zr+4Vg5eXlap5jUgGLNNPW2gc22bhp7mqHtjsaD7mB0cW84HnUG47GKjl+8UM5OT/cGF8MWp/OZaAModU1C9jDB+5jiA93U+oD2EFD7Q03fR+dnpyuDi4vycDBcp6ZZwaYJSMYOJkQFgVqU05Zh6qpuaO2RrrVGul5HgS9u+763KjyxAkqtGYZRNg2jbmJSAaRNEGhzLhQ0WAdhVkaaVgKf8Br4BMqxKmV0C+jVIbZB5goIs8UtPvew5ofhSlbIxb/++RshxkgNkpsQ4BVtQ5IKse0IZ4NbrOE49oHjOruwUpUwTZbktLiBLIvVQbEWFLTgbNu2BZtgN10hqpbFNx3HUWE76mEYrcVZevPy+gohx7HKnDEF6DSBRgsSFNcVqiu8muf567D5q7CHpTTOPrv68BOaXl8jZDM2n0m1LAs6Ogp0bsCSdizLbtiOV/rj2kGT2TsU8AAVkxhl0wlCgeusQ/KeK9wOKNgUntsWwu3BXboXxcnG9M27xat37298/PQviqHYjuUcidYszrvQuet5YjsIfEW4dlcIceh7fiPN8nVZTJe8KEFPfvwPRVO4hFjXd4iJHxBC+8C/HUWhCn4dwpxHsNn9MIrVTOZr2Wx2U1xeo2jyen4JzS68C31skvvQuRWGgUIJPqCEPobH5VEQRrtpJu/Ky8vFaDpFcQEzAUoD3oM2vA0tUGszCLxN8G4XDD6E6Hh+UEvStJS/vryZTHOUFAVCcRSVQNJVoFYCA5fjyLuVRGEZaNaAWjmIk9uplAvTqxmaSBflMkb/AyTsJWDmyWv0AAAAAElFTkSuQmCC)
Bicyclo[2.2.2]
![Bicyclo[2.2.2]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABAAAAAZCAYAAAA4/K6pAAAACXBIWXMAAAPoAAAD6AG1e1JrAAAFFElEQVQ4yxVUWW8bVRS+pImbPXbsTLw7dhIvLU0lIEjwG3jgrW+AKK2UtmmbxvF4xuPxvXP3Wb0kpZEQICqhIvqAKMsbT/wvOH440szcud/5zne+c9A7/OXen/ir2l+jLyp/eA+Mn/nzrcuxXvWkXIpCkZpeJhuT6Xhnejk1ptNJepJMNmI/2vBVvK51uIreuN/svMaPC6/Jk/xPvJubMDurJNvlgu9EcbAllEhT5pUZZzWtZVFJlReUl6knClKFW4g4/R2GnZJkOB9qnhOcFqTkbaVVJ4qjoovxrmn2DizbarvusOk4Tmtg23csy2l4VKSRMxwWCcF7lLOS8lUOMueV5B3t66MwjmruCBe73fP6xcVFwx7Y+xAt27bvWrZdx5SvIscZGBBld+QWgGpWa5UBBntSyY7S+tCjtNzv943uRXe71zdz/b5ZNPtmtWeaWdNxF9HDhw/SJycnxdPT0zL8lINa16lHDMZoU0p5KwzDyng625xdzRZVECwBs2Xtq7UgCm+Gs6sFdO/evZVHj04KZy/O9gA5Txnb8AjeEZzNGdyJ4rg8uXq1fP39D+iX339Dk6sEXb6aoZfXV4hLidD9+1+vPXt2unvRu6gOnEGRcZ7hjG5rJathFB5MZpPC7NvrVXvQR2//foOgM6nxJLkJkTKWEEJRwJe0FmkheRGyV6DmPKXUEIKVhRR7UEaJCbnlOMMUJFjGBG971MtSRrcwISkUh1FaSWkwEMvzaB2iQoiXhzIqjLEmMGoCcMl13Qx0Kw/fDjlnLdBqb4Rxbg6w5Wudl1LsQ8Ym57wGIHnqedW5kHD5EBOvAB7IeoSU5t8ApAUMqu5olEPjKM4mSVJKkrgJxukEQdiQUu8Aq6xSEkylapyLMgAXoMWVIPDrfuBXhJI5YLCJkjhYSZLIgMvNIAzu+n7Q0cAojqKM7/uGUrohhDxQStXhvBzHkRGPkzUVBjfe/vMvQkrxdchUAvT34aePIeNHQogDEC8/zyq4uA0MjuH9GM7b4IPdIIpScRygd+PPERphskhG7ibUVgcb3xFSHoEWNQAx5raGi22IDyE+gLM58DZE6rvr12hAQoS6prng4dGKksIAFlUJdXqM5QgmG9SjaS1Vydd+S2u/DeVUpJDpwA9WwyBKUT25gRzbQj5TSwyTDPMYqAwTqfSmFGJtLiToUQzDqDEXN4qSYhSEmSiK0/C8rsYvU4gztmD37XT3xXmt1+0d9i560Gu+DfOQBUNVIfa0H+5DxjlAzVfAyA9KAJoT0XQNYYw3zJ5ZPHv2vH3+4vwIxnYfLsHCIFXodQtc19FBcNsPglvaD9pg8wPQaF/7foHocHMOsNnr9XaePn1agomsdM/PC2CiIqWk6nmkjsmorrRfhwsNqXSdergGwDUOIg+oWEe+r2H+RZYQYgyHwwLYdT4DVRC0DDuhLDivgh8a0L661LrkeThHPJIBkPUe9lJodjlNQ+SBat2y+i2IW+DzgziJikEYGtCNIsH4EJjCCqO7mLLlXr+3YDnWwmPLeg+Np+PMZJJUAeDIsqxPYSd8MhqNOuC43TgZbw2dYXEwsI9gld2FrVWjyl9xB2fo3B6gE9NESIfBajKOc1BCE3bdsdnvHztDpwG2TSfjyYptWdvA6gDAW44L+5OLm/89QcgddNHZkCD02UkfvZzGi1EUGKBFB+i3RwQbLmE3vChEA8tcHmHXIJSUqOAZFcaLv/4YIq0JsmiI/gd64JBA36TWDwAAAABJRU5ErkJggg==)
Bicyclo[3.1.0]
![Bicyclo[3.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAWCAYAAAA1vze2AAAACXBIWXMAAAPoAAAD6AG1e1JrAAAGxklEQVRIx5VVSW+W1xl9PtsYAwYbGxdssB3bGI/gzxjbxBiDk9AmJB02lSpVTatWaihlTgoU8De9452Hd/gGfx5IiUmVpgXRqmO66JJFpZBuuumu/6Cq1BV9UFdd8kp39d57z3nOc55z4dnli+efXf7RV55dvXz+8+uXzv71+tXlpzevz/zlJx8MPfGctmvlWmNZm8bqg19CfesxVDa34KU/BBj94vKFkc+vXhp7+sG10c/u3D7+uJg7/tApjVcZP8SjeI+Jkh3pgydQ3foNJJu/eHmQL65faP3TT9/v+HU+d2DLcfo3fX+sGoaTKSHDVqgeae0e/X8gn7w8iNqIWklsuwJj+zyhB11Cxx0/HHX9YIAw0a6U3m7TSqPZeATpR78Hu/kYbjx/DpWigD88/BSkVwJKCAhOwFgNUWzBJmW4tCLhwl0JBRoBrMW0zSp+QCs9yKgY893gWDFfHFm5d68nt5JruXjxYoZLAc/x4h87j+DGlQjUSgnEzTvAAgGK1eBu7ioQ4iCIgSSJIK2tQ/b178PUuxJurFCAJOK7lULGUnVzxgc9z5sqlkrTuUL+SMl1O8MwbPrPv/4BlQ8/hgKrw7ffewKk5MGnX38XLE/gfvURXLr2PXCdlYwyJmMRKK6uw+T0WwBTefjOD24ARJaC1Rrux3GToryLhWSYhOR4SMkxIdiYMbIvSWxHZW2jRX34W8hd+i4kV26BvZXLpCptMiHfKbjsVJL3oFx9KFeXiZNdN4MkcyVvgEUpgsRJQ5SUG4zgLSwM9weeNxIE4athSE5yzua1kRM2MofSer31G8nf4bZDwF1Lwc/lm33Hbw/d4BAJ6bAUfNxGejJO436bpO137FbG4xyYEQCMq06h7D5KZZfn+q9gP8Zdx10I/OAMp+QNJeWyNmZKmqi35AVtYei1Eyo6fcfrLhXc4WK+kPVcb4ERsmi0WoriOIuV9PIoabGxyFAtG0Ao2YuXDAkhR30/nMznCrjy806htED98DXJ1Bkt7RxjatgPgm7Kgl4uxBGUdMJx/Bf7Z0rF4lzoBSeVkHPWxlll9IiQ7CCqsC+p1TpBadlrjDmMjR9jlE/4nj/pe95CiJUIys5paZattqeENOOEkj5KgwHO+SQlLIsyHfP94AQJgiUW0HOCynNKmnku5BgXtFsb1VVeXd2HlpO74kS3R1p0KykOcxpmGWNnGWWLSuo5BBiPjB01On7xv43zsE1Jtl8K2a+lGNZKzgkuFiVXpwVVJ5RQRwhhhxgLO4xRHZV6fS9sblQbkkrUmGqxOzZ6CA8uSSneQUkWhTZDUTltK1ft7sparcUmURNnfoORwQ4aBj2SkxkEedto/Y5SZomGciT0WcedO6z5480Azt3+1v8m/t57d2GzbKGs2fZIqR6j5DSCLHLBs1zJfhNHHeVqubVcrTRXV+uZMHSglroNgV/ay2kwKgQ/rZRcRolmKaOH8VxXlMQ7dOV+Y3X9z/DNCz8EKJerEKqgIYp0a2TModjGo0bqY4rLrOB8Ak0xGFfKe8u12vY9OwGqVdIQx6I1JF4fZ/SYYGLOSLP0QjLs1QyXsjdJ013R6oPGtU/+BuW1+xgr9fVtcRK32sh2Iat+QuhQ4PrjxA9nOGXHldbjcSX5UlyOW6Xi26Rirdro/ch4ABs/gvYdD30yR0Myj6RmlVaDWH27rDxoNMkqVNY3kFmlti+OY3SYHSaUHnVdL1vIF2ZLhdKrgeef4IxNGaMHtTY9UqhOqcQBDM0hxvlR3wuzxUIRLewsBK43yymd0VqOSKX6KRedXOkdydpmM1TKlQMYaoctDhxjfM51/WkEmUP/z/uOO88JXUDpFgRXU5TwPqlVj1JqFHNuGgc2izMy/QIkdL1F3LukhJgRUk2gcXpNFLena5u7IE3Tjji2PRhsA3hwFIdsLPT9WbzgFOYYWlPgQXVKCI2zIbql0Xux0egsMYjMRxkl02j3szhTb0kmvioYX0ITHMVKD5oYQeoIIq3XkKZyZxzJDqPUgOTsKObQGWziWQy+OSn0GFYxwKja7zhkp03NNmMlPg+iTytxFNdpKdWbCPqmYPI0C9kw5Wof57IFzdJcrm80gcaU/ONDCzUbNhspDmohZpHpecysLwuFEgnTRVerTVu/uw/Pbz6H+mqcITTYQQLnAKPhFBJ6Q2v9NibGaxhNExhNXS7T27Z+vg5Cx1BdR3e9X4yg7+IDqBp/J+o5aCRmlTJfQ5BzBC1c9Pz2W4Rm4DPAh+spfPSzWgbtu6dUWnnF971ZRumy0fZ1TICTKFFfrpTfVfvnv+FX5iZILKC6vgn/BT94i5/7QX/SAAAAAElFTkSuQmCC)
Bicyclo[3.1.1]
![Bicyclo[3.1.1]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAABnElEQVQoz52RzUobURiG39ZIpcRFIqhtUDRxJbhS2jvouhsLBSniX8cmk6BOEiZzMnMmkzmZ4+TMj/G3VVpiV+qiN1DcuHEhmOjChXfQm2j0CowfvNv34X0+tOXVubYsfW7lMl9ucumPt7nsh/t1eepqIz/2h/FBVt+NesHeK2/3J3jjOzRNQ7FYRFfXkldnWrI03c5m3l2tZacv8vmZv5o6eUL0iUPmvGGO139w+DtCqxyb2z/QbDahqiqCIOgCkv4au06vxG8zUvw8rwyflY3xXxUrFdJKkhnmCCH6kEb06NLC/Aub18E5R6FQgOd5T4d0dOHu2zIulA0s7hxHLC5iNS5GTctOEZ2m1JI61lE01MlrwzB6CCGPysIw7GoJLnNp/JubxXHNfembdp9gbswyrQSlZrJcLid1XX/bAUQppZEHwEOEEN39JagJeNSBT2zw95/Q4Fu9TtUZcNhmwrZZgtksblWtPtM0e0ql0vMgIfexH0mhcXoCv8KxIwIIR/T7bhgP6lux/e2jXiAKxhxIkgRFUeC67pP7/wPslaY8nbvs/wAAAABJRU5ErkJggg==)
Bicyclo[3.3.1]
![Bicyclo[3.3.1]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAABe0lEQVQoz6WQu0pjURiFfzyixdgkwomMoI3aBBLGy4xo4xvY2AjaakxycpQ0GTGJyb7+e+9jTqIoqbz21lYKgmC5RcUHmQdwY2mVMAs+VvmxFrwEOxsvQW7dhsVNWyqs2b1w9bW8++dpvzJzi9KvkkaiUa9N1Or1CcJFynSvBqPuNYj4FLTW0FNssDNvi7k5Wyr+tqX8LyfJvJXDyft69ee5NuMo9ZRgIsson+YCfXV2OaC6N8CiTh+SwnbCFraSNsiPOpJ2bzfxUQ5/3Aky0pZqTHKZZoQvMsLShHC/waV3yBFqTdq7xN3l1uTA3QXuLngPA3is/IX4/MLjVPjI5axguIJCzQlhxghtDDbJIZBms68lji0nyn/xUKkAPP+DY90ZUlL4WsgMClxCqTIcjc8Y8SgjQBnvXfI9Ld0Co45dt4djo1MRqmxkzLLWUdbE7RRF6VFEoBL/QxLFELe7EOmWdxKbZKRw5ujILKDRadOJ/QNCvQPCoNrHkk/mjbLT+3K42wAAAABJRU5ErkJggg==)
Bicyclo[4.1.0]
![Bicyclo[4.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAACiElEQVQozw2R3UtTcRjHf75mtonOHXVO52ZzbSPdMumiorovuuom8KZ3X2a6KcHayzm/95dzds7mHKhp0kUEUdchUVARGISpdN3f4d06D3x5ePh8ny8PPOB4aX72ZGnu/snzxdm/mWd3jpYW7x6uZK79zGYT3/MvvM29baAvLLbCEmzLr611moL4lFBRKcwop47/9q0bLVRHfRxxjSE2zJkICCqSSlixinI0498RACeZuZk/ywtXDnIrN3+sLl/9kstd3y/kZ95DI71nW2O6bXsq0unmWHiQDv2M4KDg8oIbEpG81tdsNoFJpcflGoFkjGISFUykTGXFLKvW3wRuHaxlBj7p+cgHZKTeIpjaLZWndzCaajCablSdcWFbPsuy/RiSIDJwHEEYZ5QnJVchaFBtYX6+q1wo+V0WQAYKE4zinPG0kmbMtGq9p28/AvDw+HWrerPtNRuNUeGsx3TCLyLGk5TzhLNeGzUrls9xqhrFLIIRvWzo6BJEKGkYMJDLrQ48fvK0J5vNaqViabBcLocwMuKMs4RUMqSc9e7fv/YBeFcvdW5tON5GvT5UtZ3znNIp99qUrusThBKfEOSMY5seTtgQJTSBIJnEGE8ijMeLxZeuCuFCsRgxEAoaEI4QgiYYp1Eu+ZAwK53N5ikAW7VyS+1VHTSqGx22svycsnE3JOkuhBFGvZCS9q2qaK0qq1tyrhHCgi4PYUJGoFEahdAIud4AIlSDmPQTioe5YINCiR4pzbbpQ/crOxuPwO5mA+zV11tqlupRUgYZo3HM6BhhpN+sVdulJcC9Bytgs67aTFN1cCm63O51LO5Tko+48yBiohcrq4tLdk4ofpYr1vH18zcQTs+A/4rOKzVeROqcAAAAAElFTkSuQmCC)
Bicyclo[5.1.0]
![Bicyclo[5.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAABlElEQVQoz53Py0rjYBjG8VeLJ1p0UERbdSGVKYMizHgAL0NcCYJLq8ZYUZlDbPN9icmXnpI0TZpTrbGCRRnGgGsZmNm5stXdwOy8jtqFNxCf/Z8fD7T2tjeae8n15j612aR31h5pavUpRa0809T0C3M08PcUAbTbYHk3cKYWoVYgoN/+gUBrUcnFFrW10KJ3lx/p3c8PhwdL98y3Lz5iP3mF/IRsOYOu4w6YZ42QVa2DbZ9DxfsZDOm8GP59tB+9+/E1fpNJJxoYz9ZFca4qSTOmosQ0yx5y3DfE8cC2amBc/AqGpF78LuLoEaJrUaKVp0VZ/ijm8rMCkRLZXH6yVNYHLdftN2uNkO164JhVMOp+MMQ3UI+p5oZKcmFSkZWERLLzAi/OsRk2jlk0zJyc9B6m6K58sQRitgjGpQ+mYgRDrmwSsipan66XI5pmjBAiTXEcH0+nM3GWZccw5sI8z3cz348BZdLvQ65dHqyyCoxKwCgYfZKQ/SDwwjhCKIYxHu2AYYxQN0IscBwH/zuN+q8dyHgFntCs5PPvjVYAAAAASUVORK5CYII=)
Bicyclo[6.1.0]
![Bicyclo[6.1.0]](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAABkAAAAHCAYAAAD9NeaIAAAACXBIWXMAAAPoAAAD6AG1e1JrAAABcElEQVQoz53PXStDcQAG8D/HLixTXhrFUGvlrbyklMQXUK7VkjuObcfacDbO//1Mxw52XpG4kHJDyhVJilIutNiu5Nqlz8DaNzh76rl9fj3gMyFGS4mVhVIiFi3HlufLMXGunIxPfaRSkXdGA78wCb7QBlhcmgIIoWo9pxRfmSiurU6+pZOzLxupmef19PRjRh57kDM9t6oW+OYi+DsfBYztAYwxEEXRO1Jcl9rvt+XeG4qHLzkfv6B05BTTIVeBoQOitohZVl9gWUAorr6QJMk78lMpOTvx68eHQd1ywnxX70dUHd6CKIIw7SSENmIMhYy8CRRFAZRS78jr1YngGPutrlXoMwvGkL6rDzLKByCEEYxRd2W0mWDcAKECCCG1IcW70zrHMZpc1+wwLat3T98Pcb7ThRDuJJS2Mcb9hGAhW3lSM/J07TQcH9ktrmuFTNsO27YbLJhOU05VfYxzH2NMyOfzwDINoGkayOVyno1/osuZEUynW9sAAAAASUVORK5CYII=)
Bifluoride

Bismuth

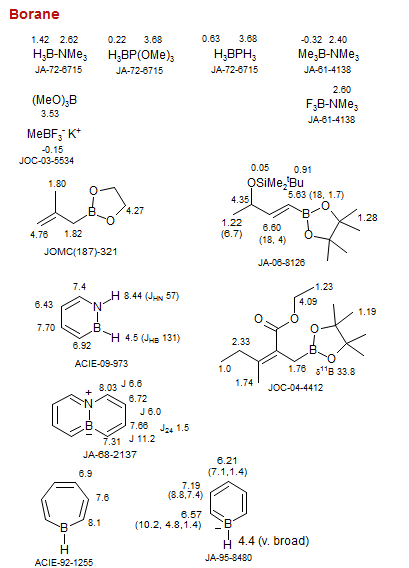
Borane

Bunte Salts

Cadmium

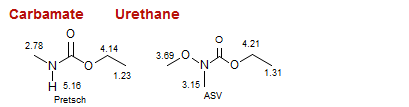
Carbamate

Carbene

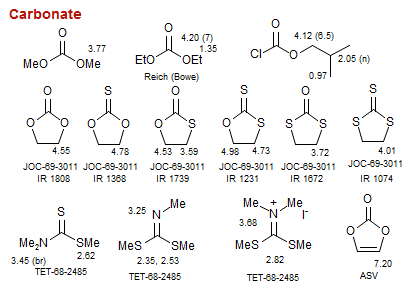
Carbonate

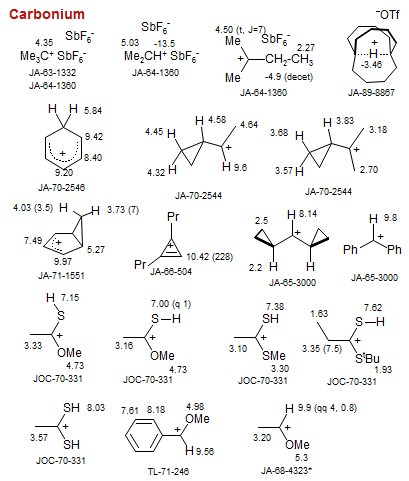
Carbonium

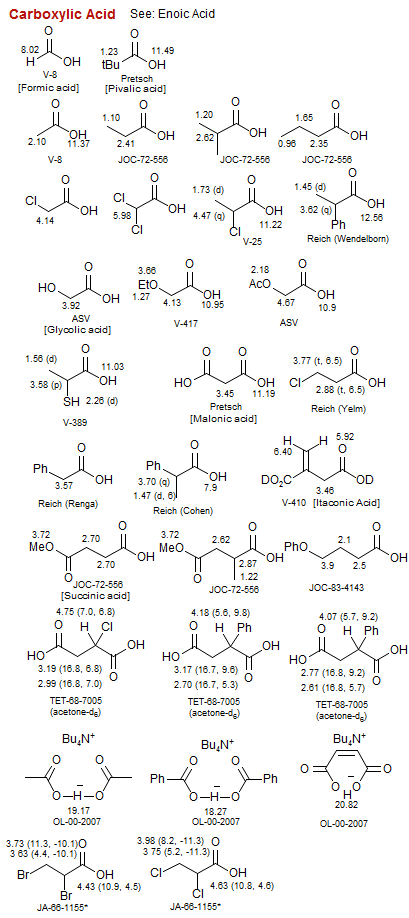
Carboxylic Acid

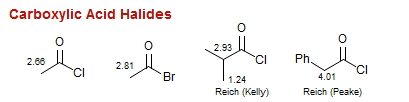
Carboxylic Acid Halides

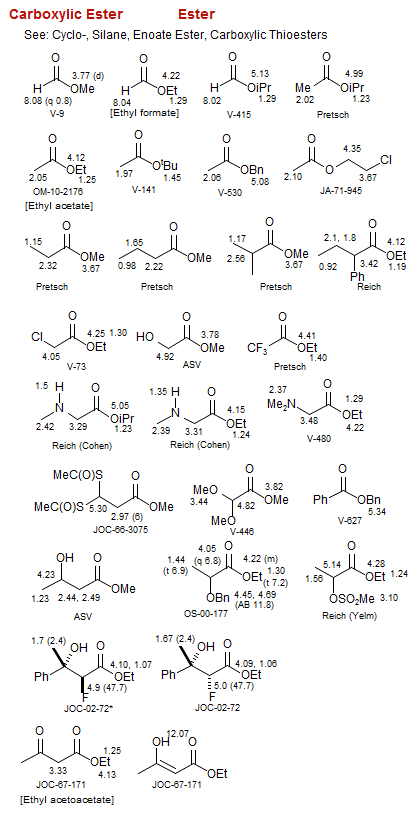
Carboxylic Ester

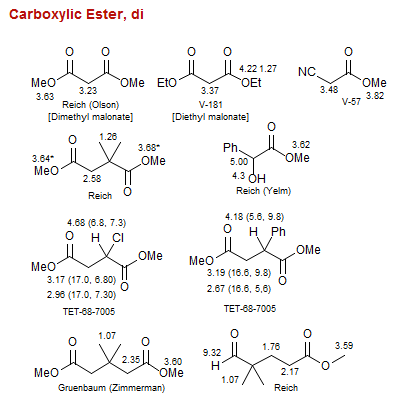
Carboxylic Ester, di

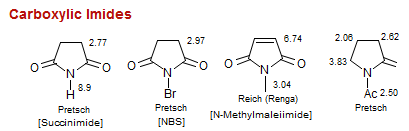
Carboxylic Imides

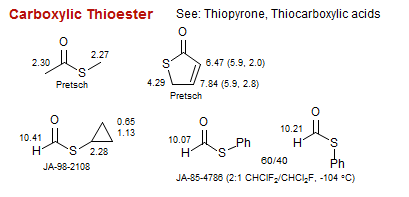
Carboxylic Thioester

Copper

Coumarin

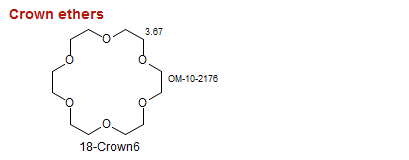
Crown ethers

Cyanate

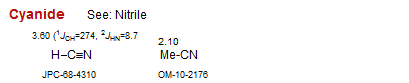
Cyanide

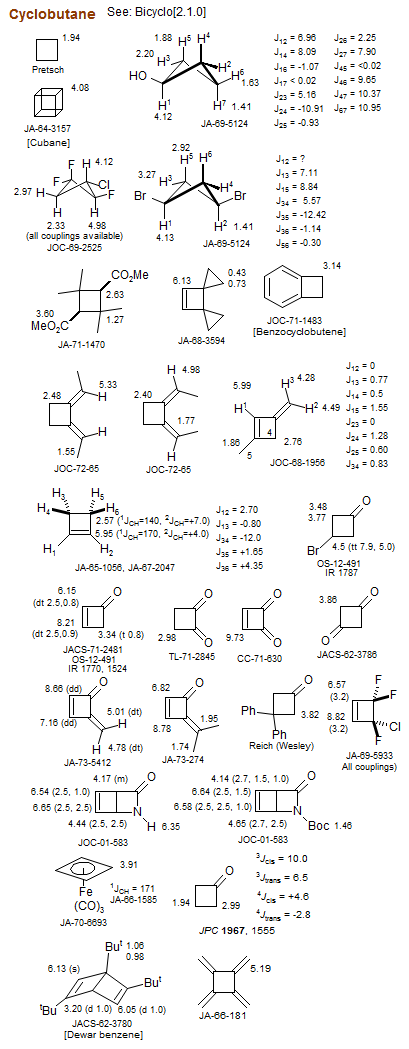
Cyclobutane

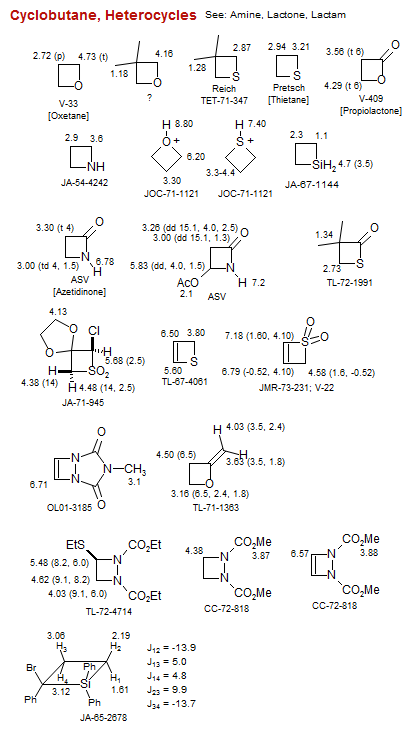
Cyclobutane, Heterocycles

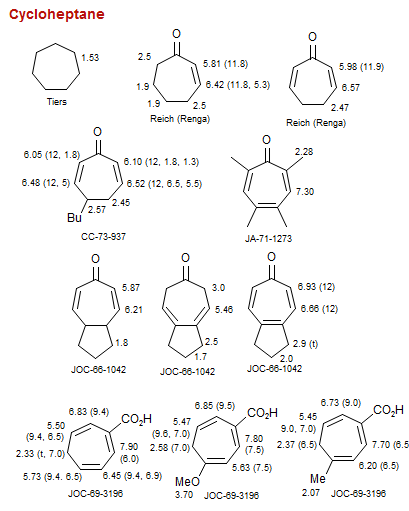
Cycloheptane

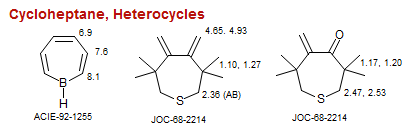
Cycloheptane, Heterocycles

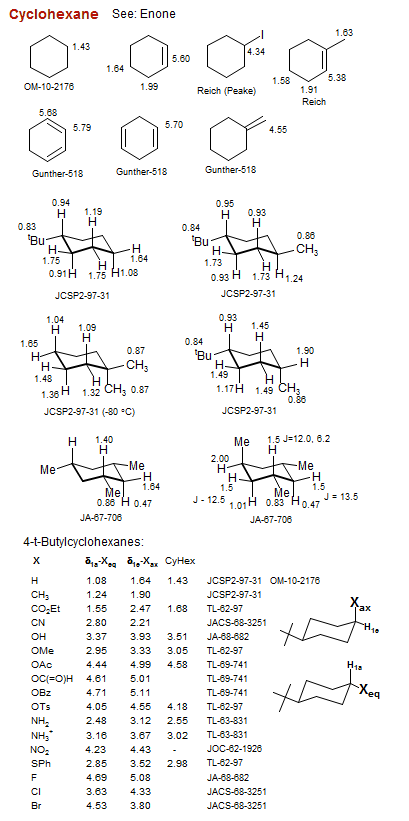
Cyclohexane

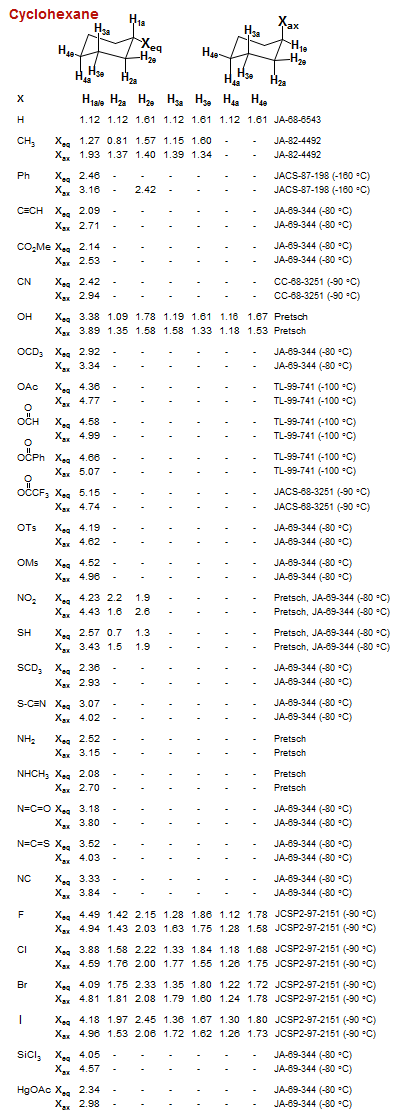
Cyclohexane

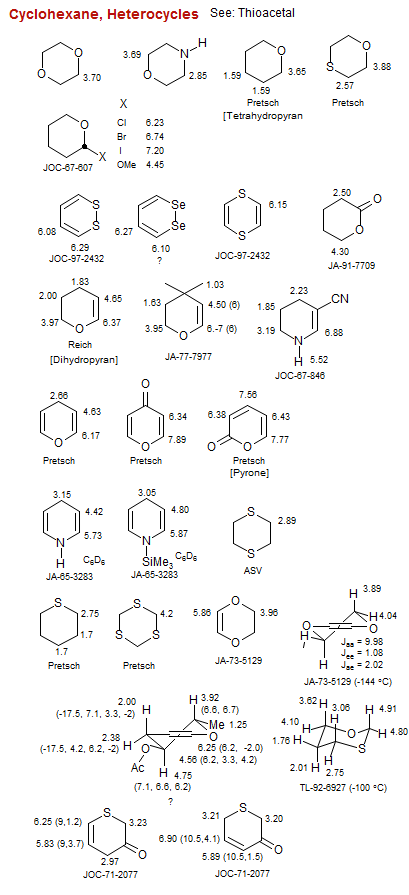
Cyclohexane, Heterocycles

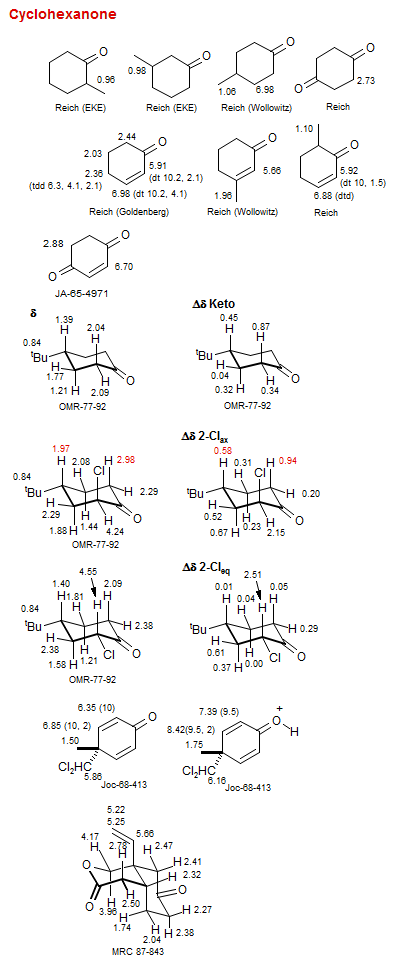
Cyclohexanone

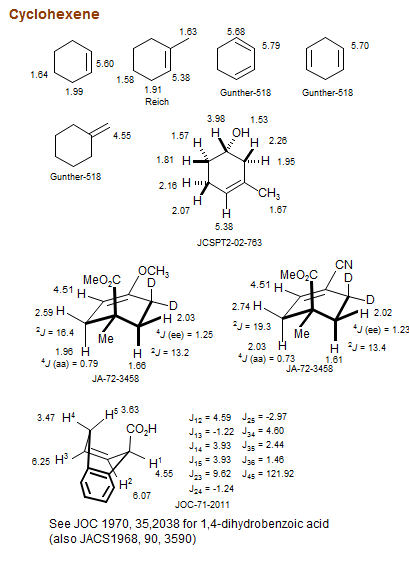
Cyclohexene

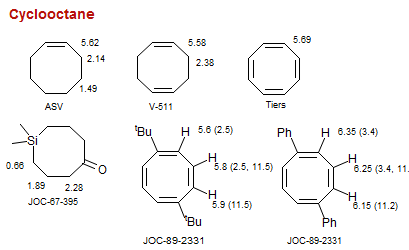
Cyclooctane

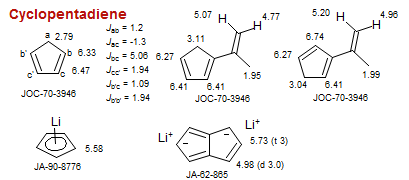
Cyclopentadiene

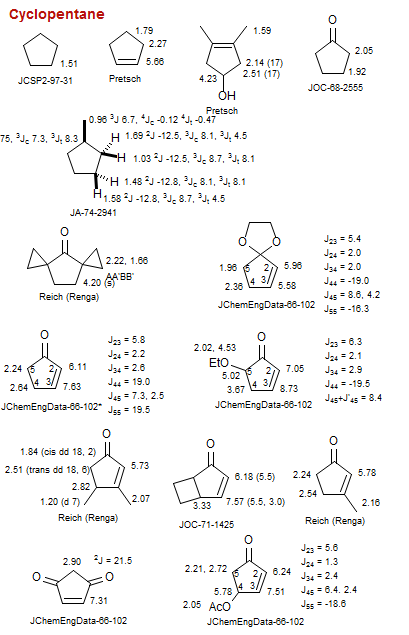
Cyclopentane

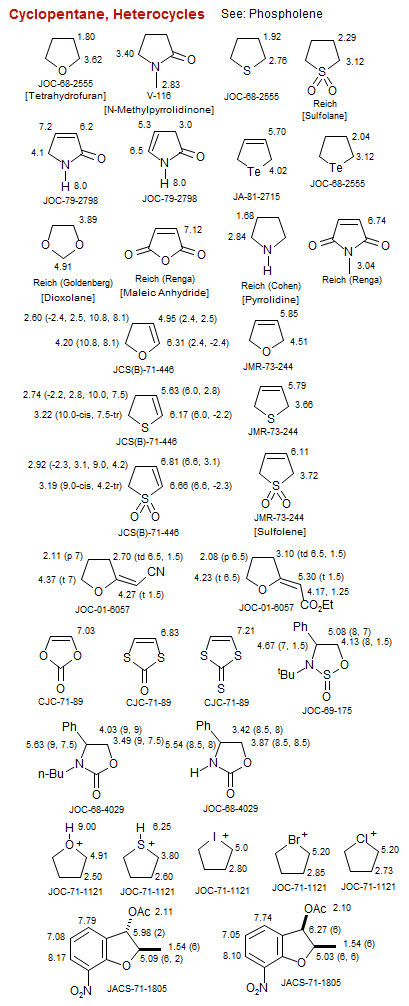
Cyclopentane, Heterocycles

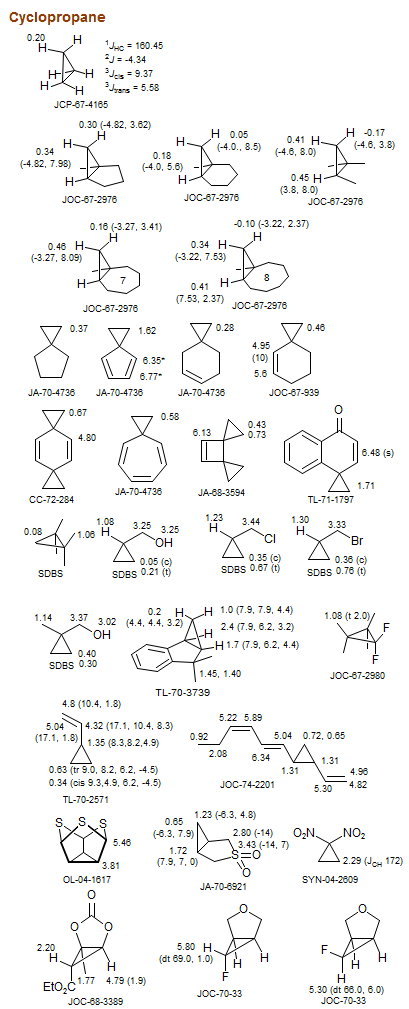
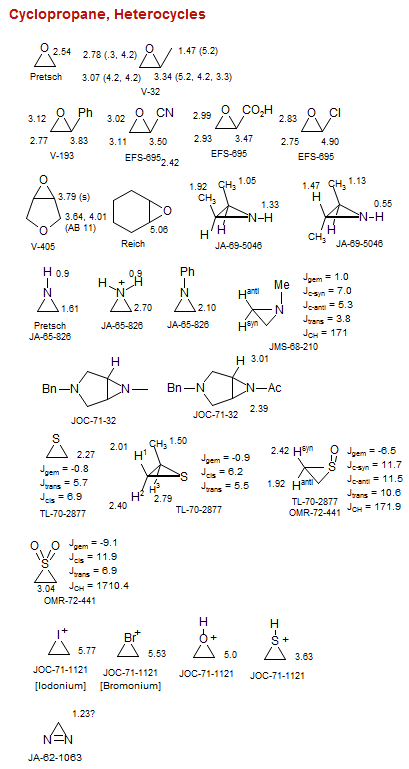
Cyclopropane

Cyclopropane, Heterocycles

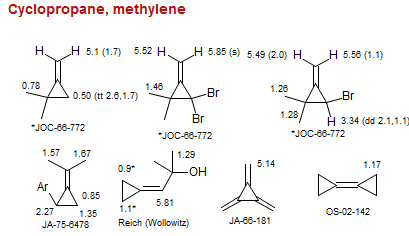
Cyclopropane, methylene

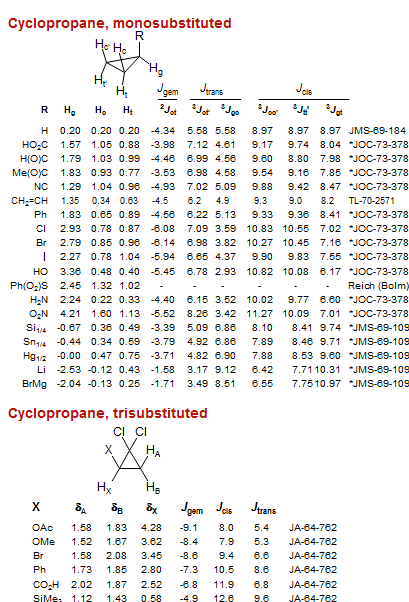
Cyclopropane, monosubstituted

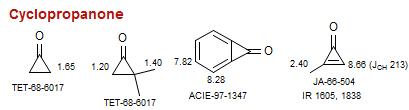
Cyclopropanone

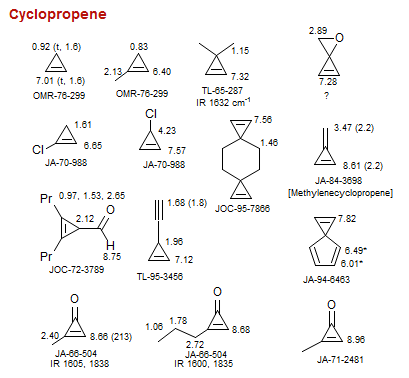
Cyclopropene

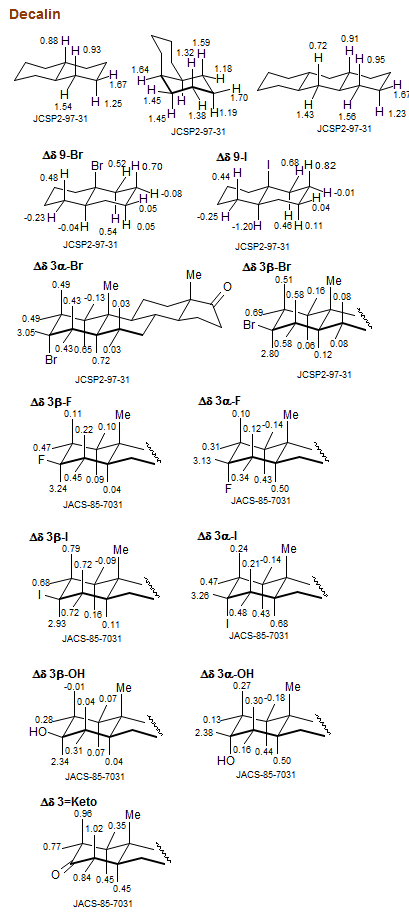
Decalin

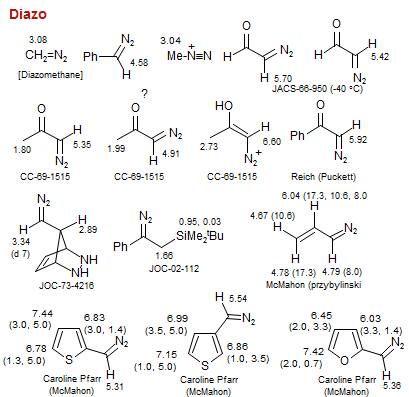
Diazo

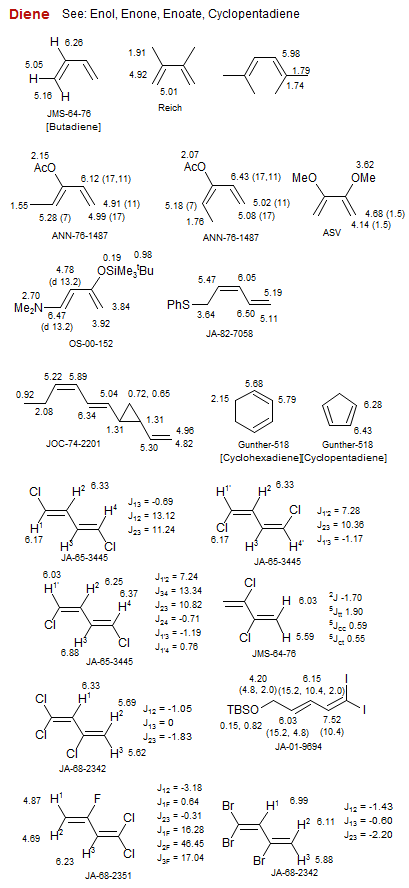
Diene

Diene, 2-Substituted

Diselenide

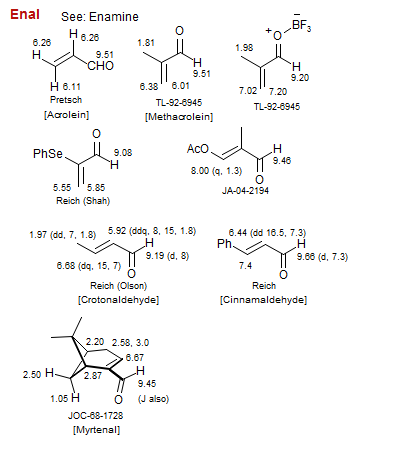
Enal

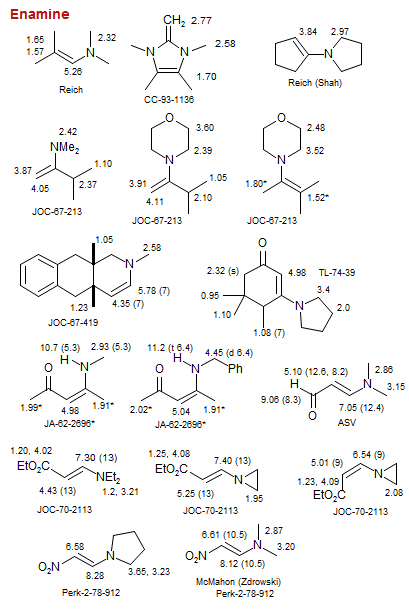
Enamine

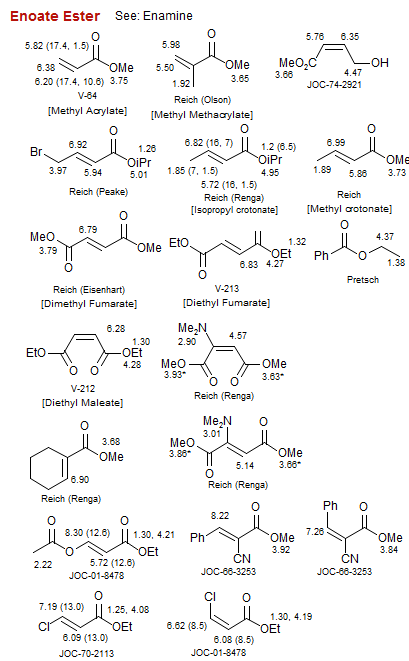
Enoate Ester

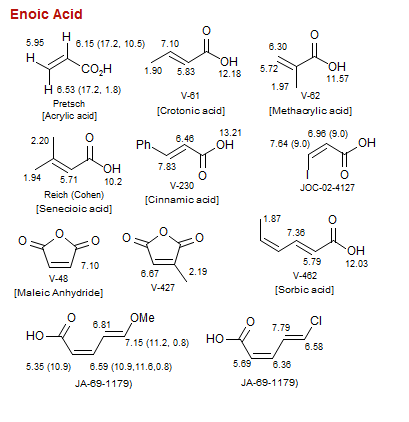
Enoic Acid

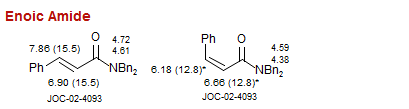
Enoic Amide

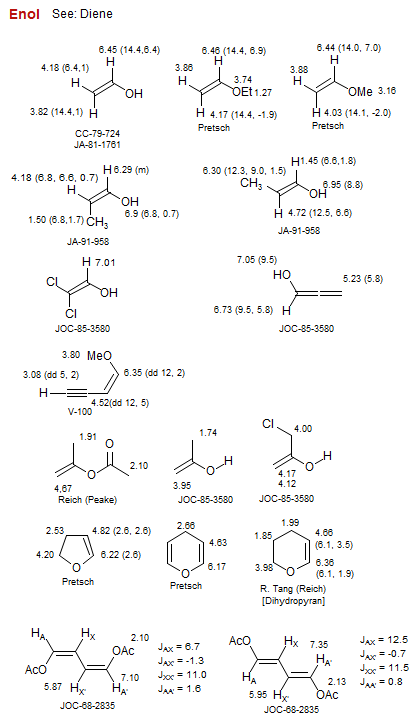
Enol

Enol Phosphate

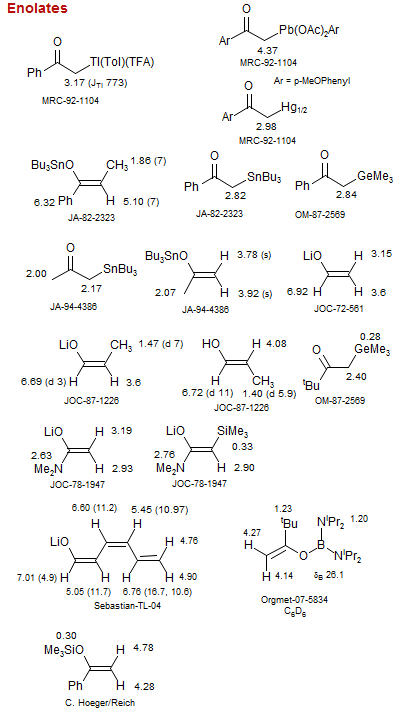
Enolates

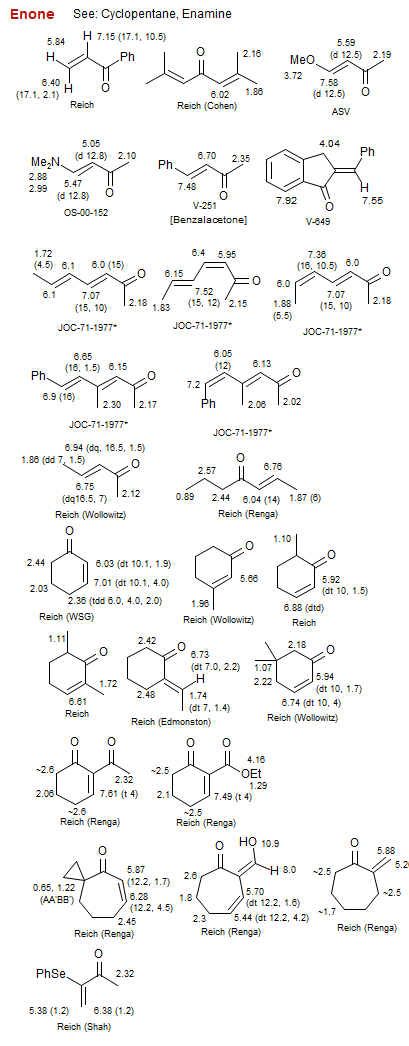
Enone

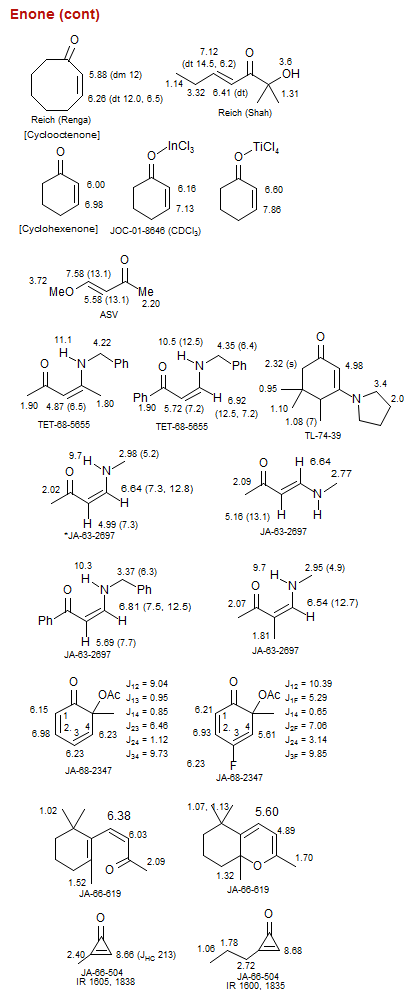
Enone (cont)

Episulfide

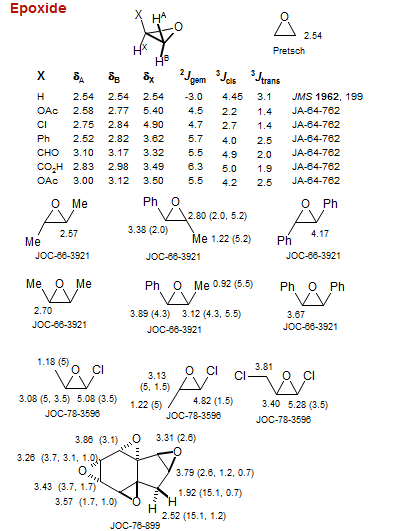
Epoxide

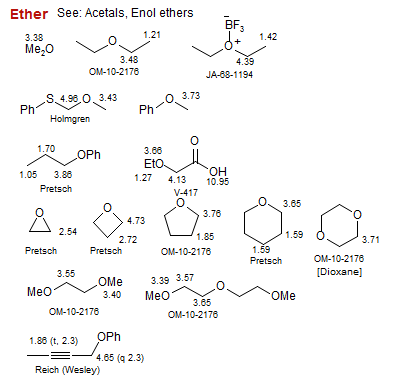
Ether

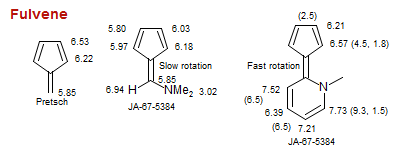
Fulvene

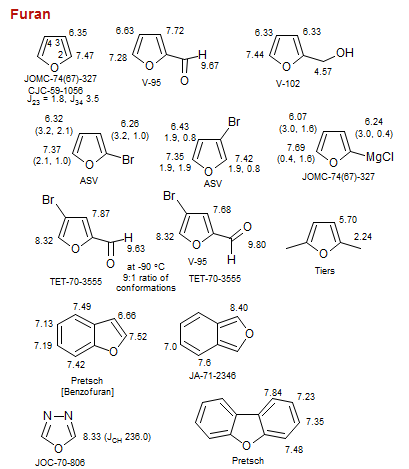
Furan

Germane

Gold

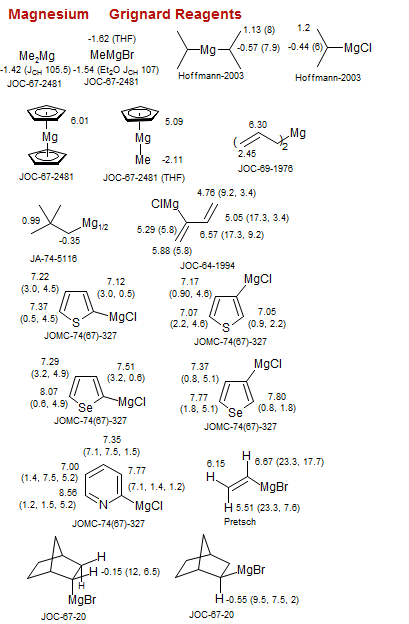
Grignard Reagents

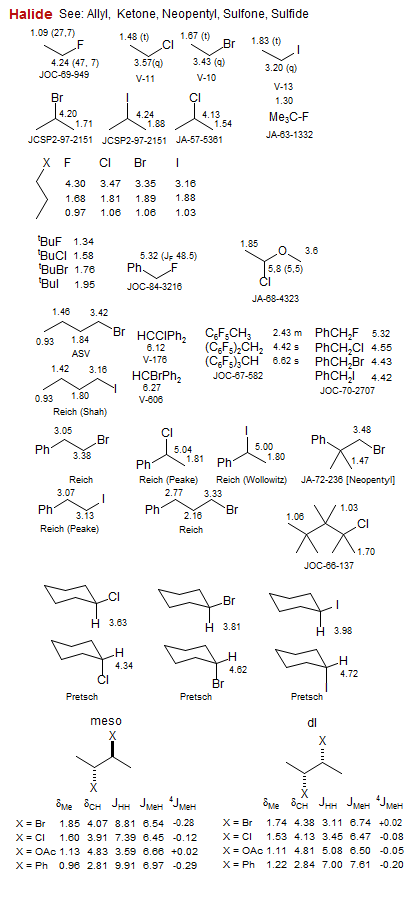
Halide

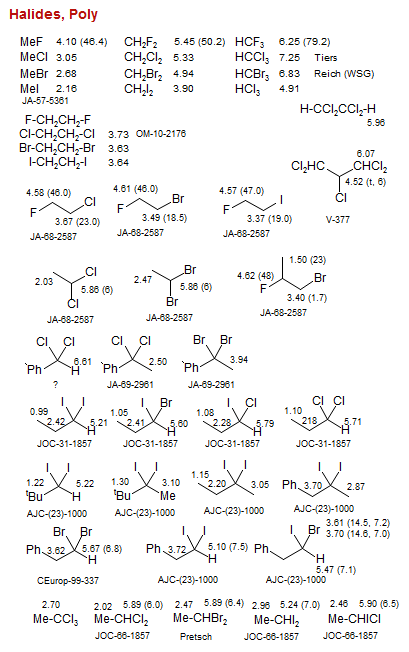
Halides, Poly

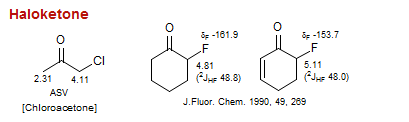
Haloketone

Halonium ions

Hydrazine

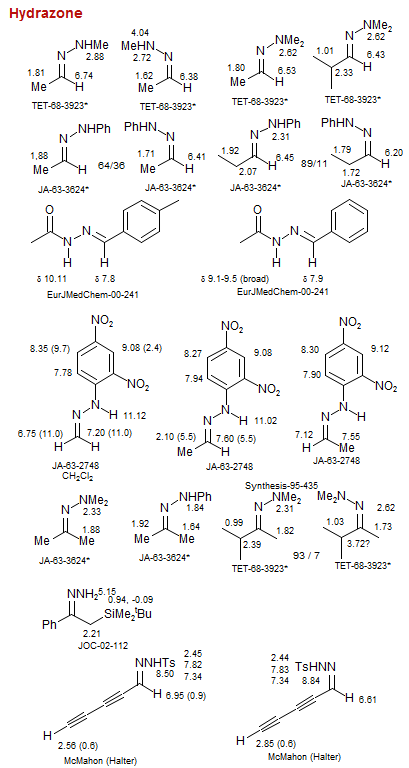
Hydrazone

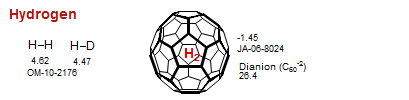
Hydrogen

Hydroxylamine

Hypofluorite

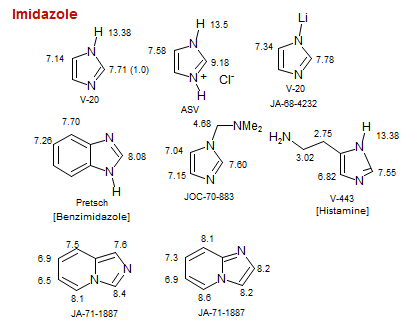
Imidazole

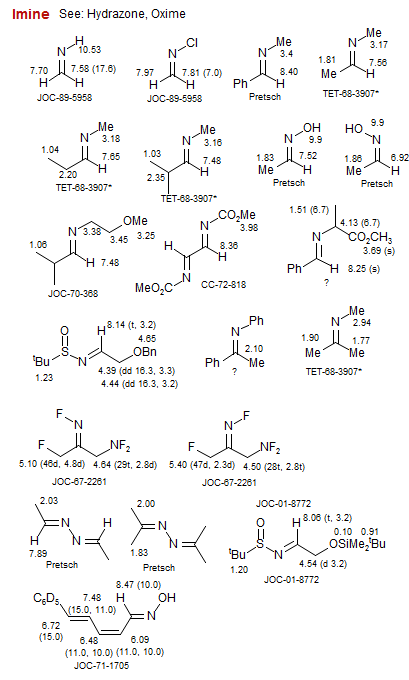
Imine

Imonium

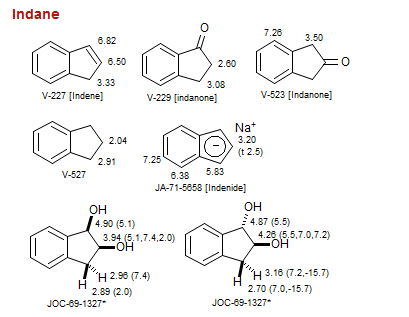
Indane

Indium

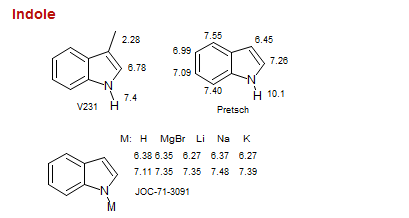
Indole

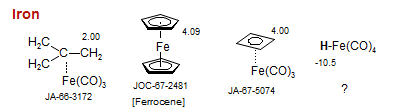
Iron

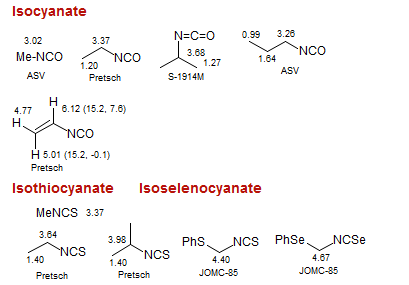
Isocyanate

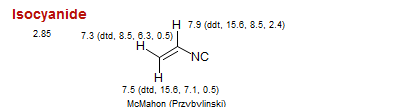
Isocyanide

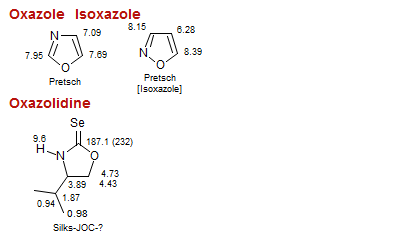
Isoxazole

Ketal

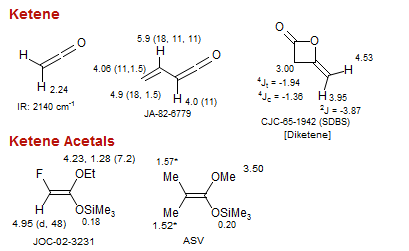
Ketene

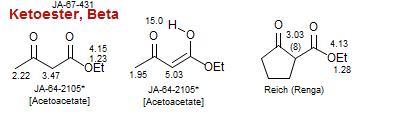
Ketoester, Beta

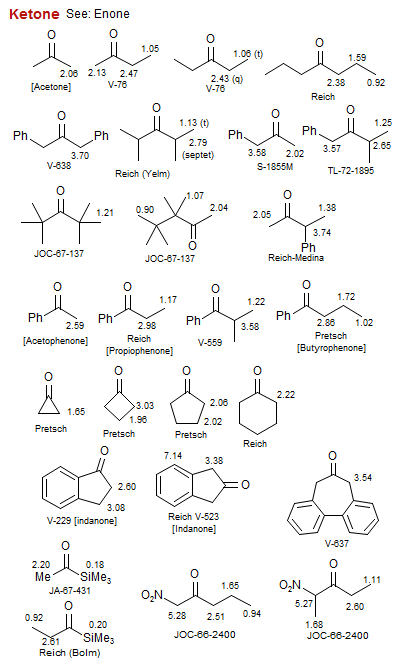
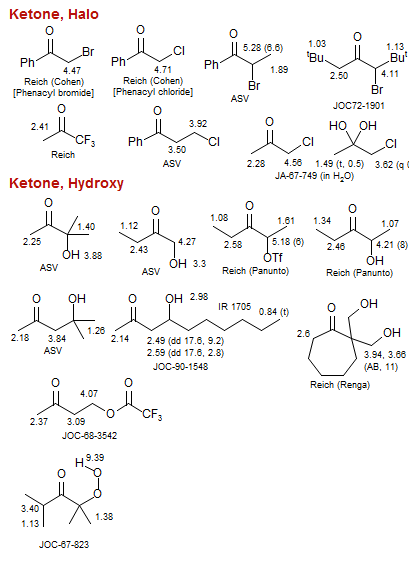
Ketone

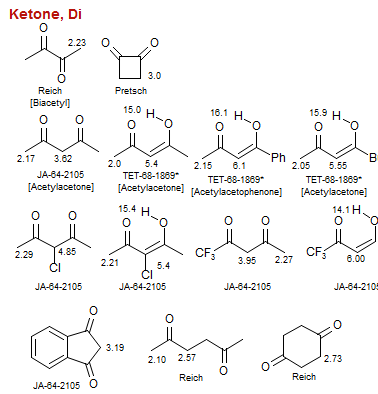
Ketone, Di

Ketone, Halo

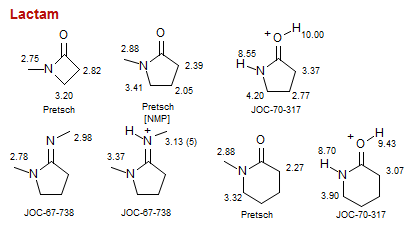
Lactam

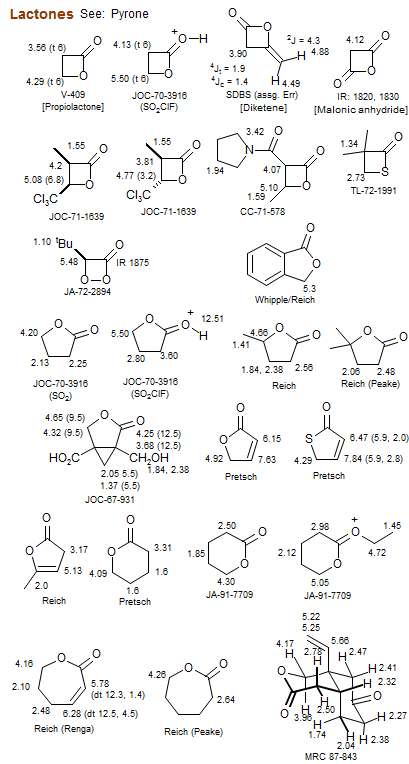
Lactones

Lead

Lead

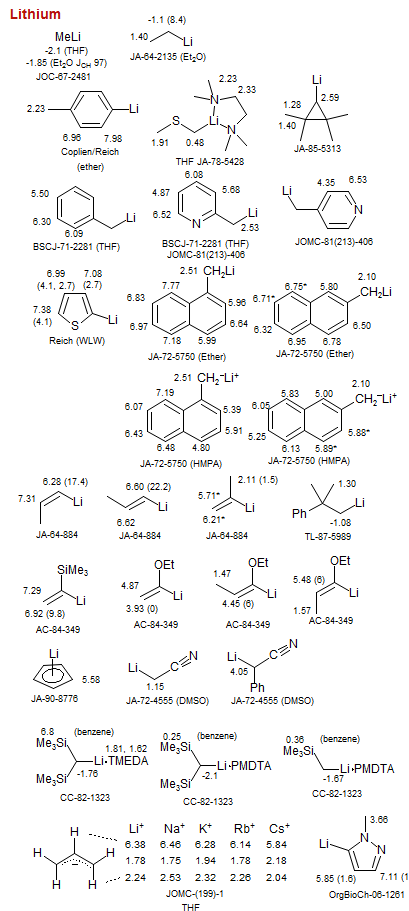
Lithium

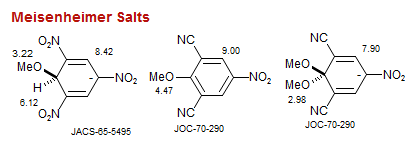
Meisenheimer Salts

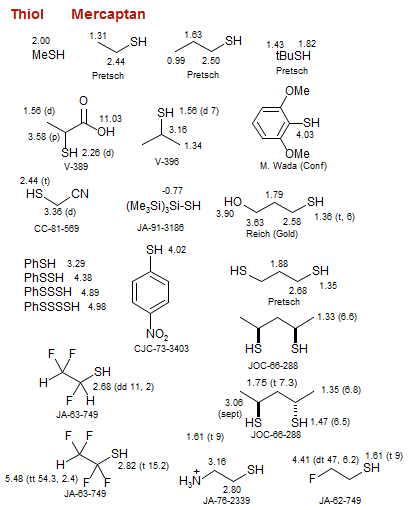
Mercaptan

Mercury

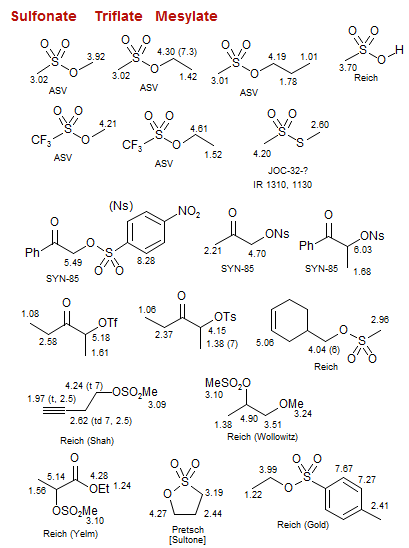
Mesylate

Methyl Shifts (C, Si, Ge, Sn, Met)

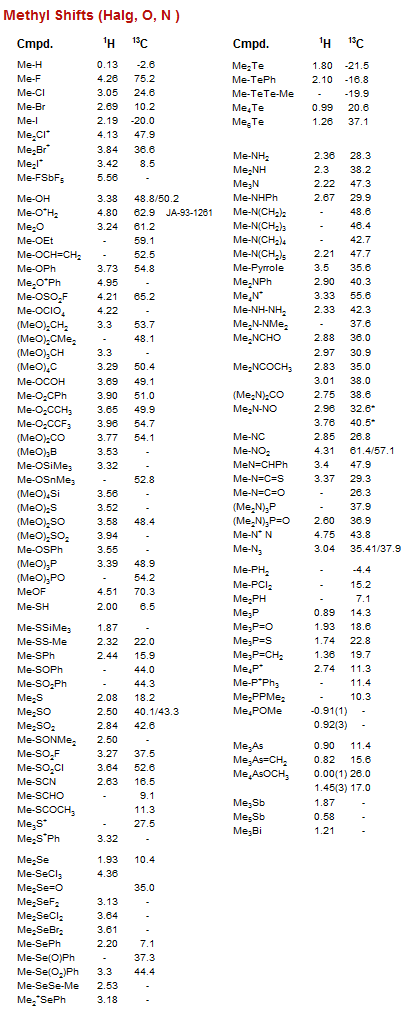
Methyl Shifts (Halg, O, N )

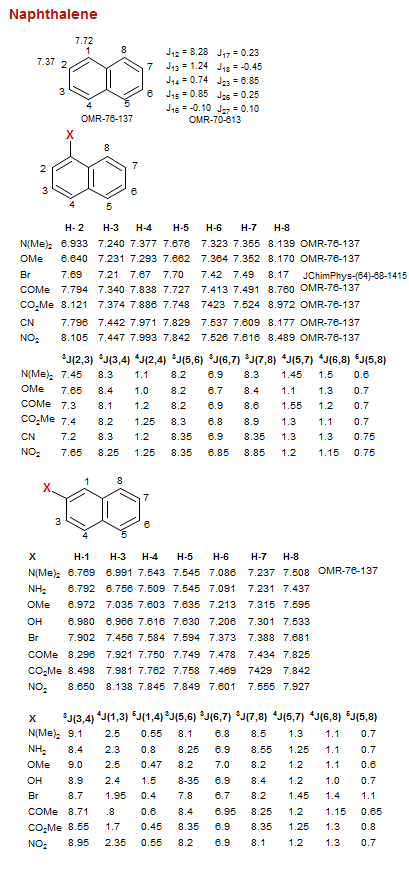
Naphthalene

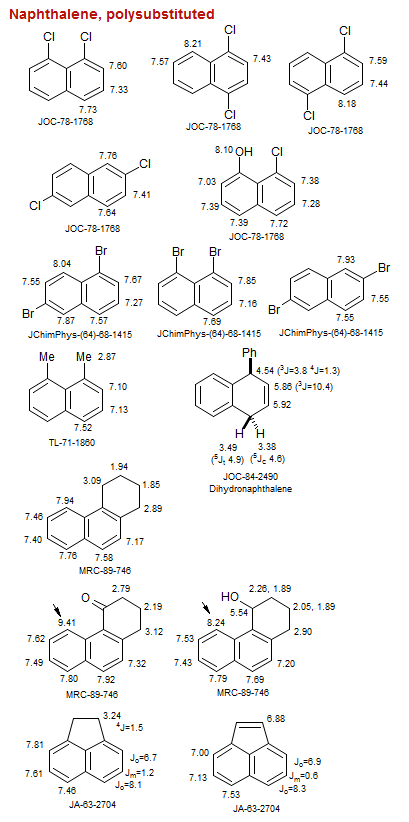
Naphthalene, polysubstituted

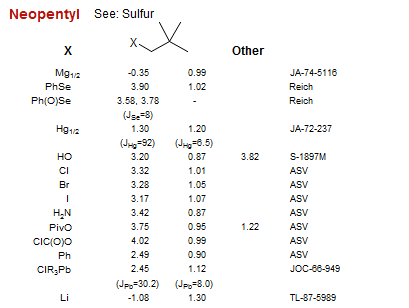
Neopentyl

Nitrate Ester

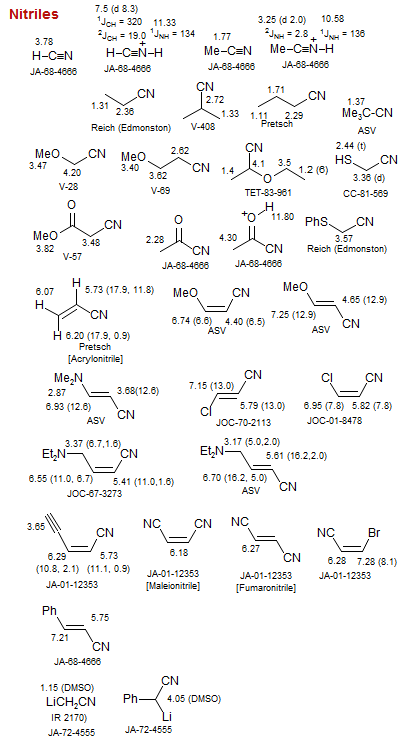
Nitriles

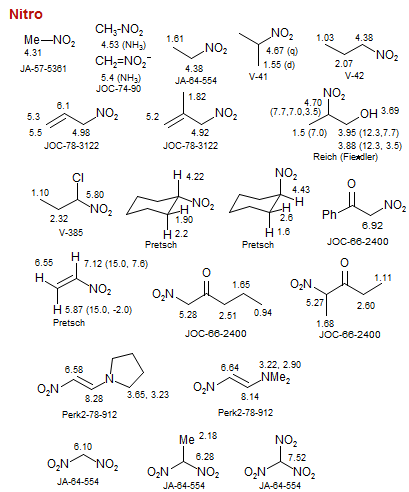
Nitro

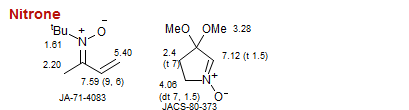
Nitrone

Nitronic ester

Nitroso

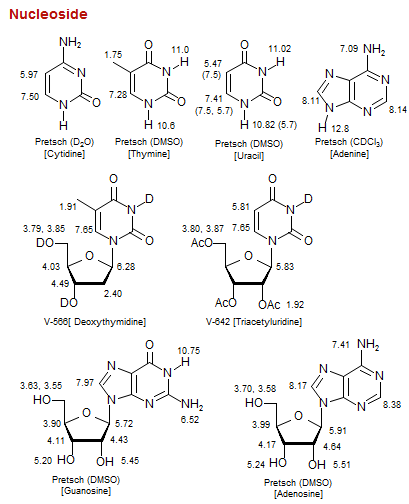
Nucleoside

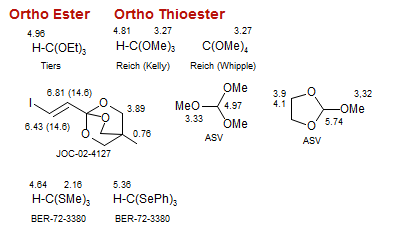
Ortho Ester

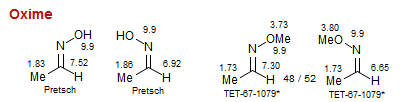
Oxime

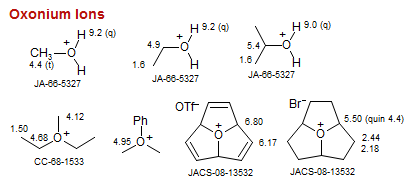
Oxonium Ions

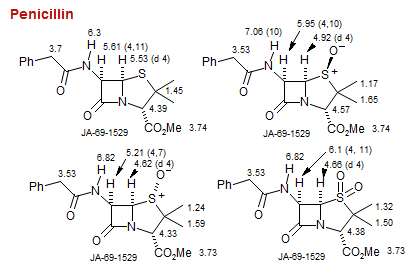
Penicillin

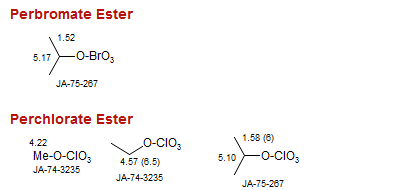
Perbromate Ester

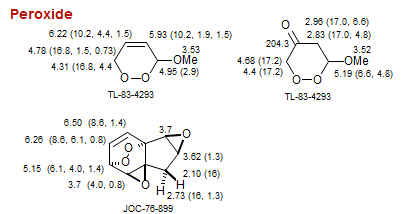
Peroxide

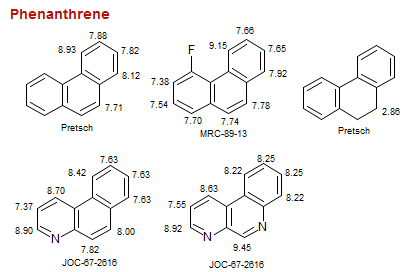
Phenanthrene

Phenazine

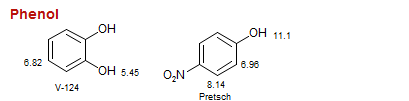
Phenol

Phosphaalkyne

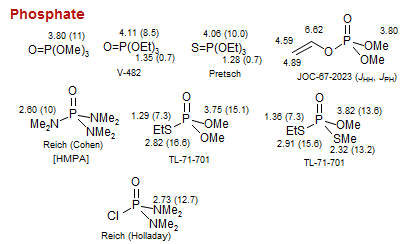
Phosphate

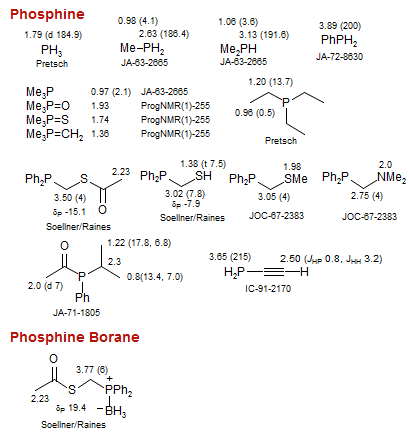
Phosphine

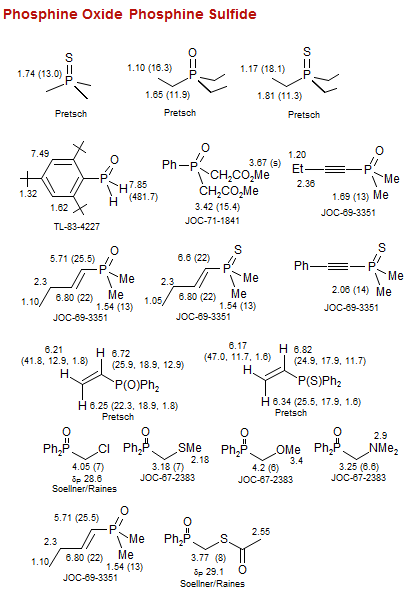
Phosphine Oxide

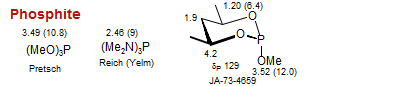
Phosphite

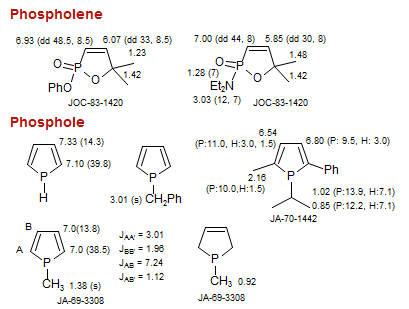
Phosphole

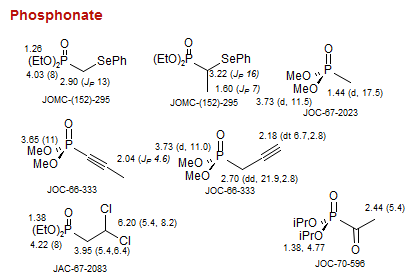
Phosphonate

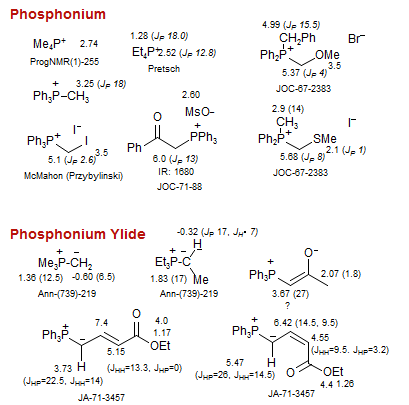
Phosphonium

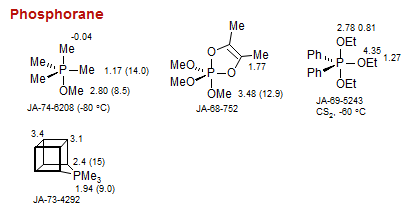
Phosphorane

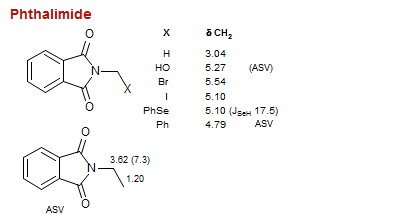
Phthalimide

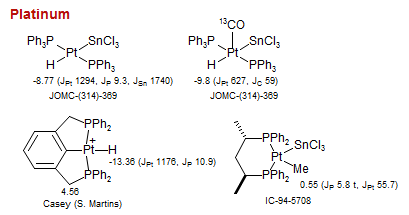
Platinum

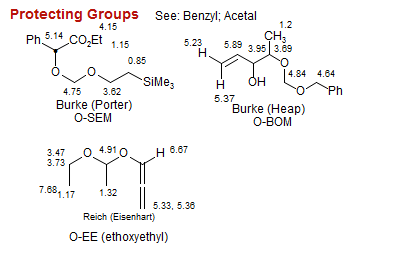
Protecting Groups

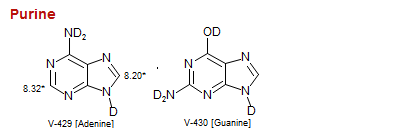
Purine

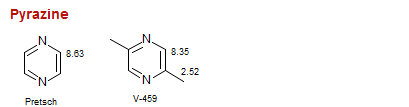
Pyrazine

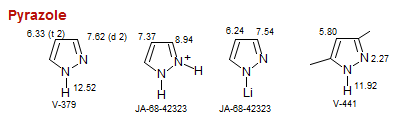
Pyrazole

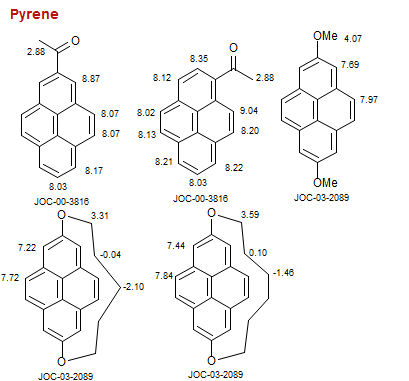
Pyrene

Pyridazine

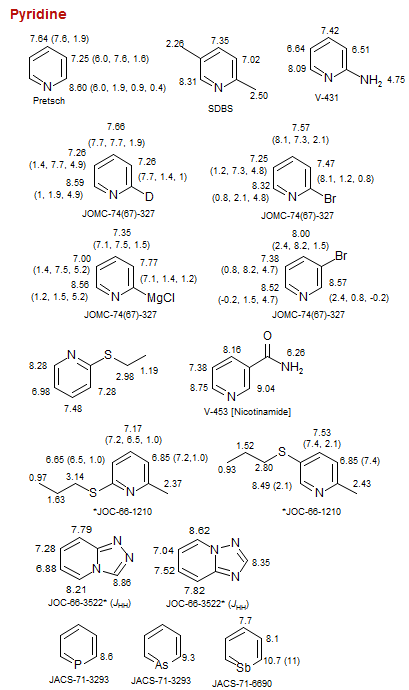
Pyridine

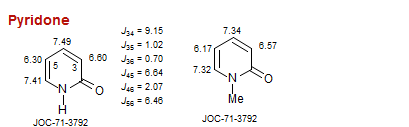
Pyridone

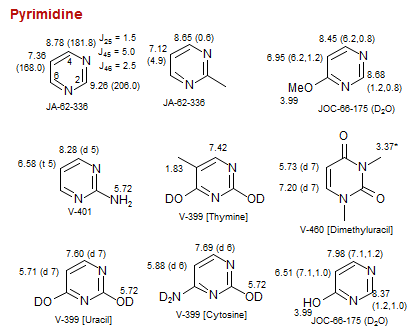
Pyrimidine

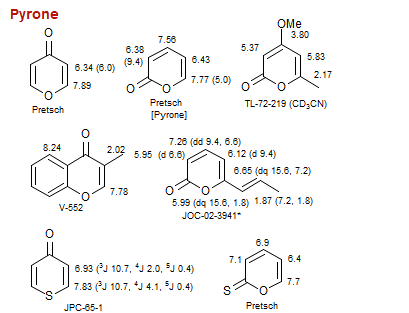
Pyrone

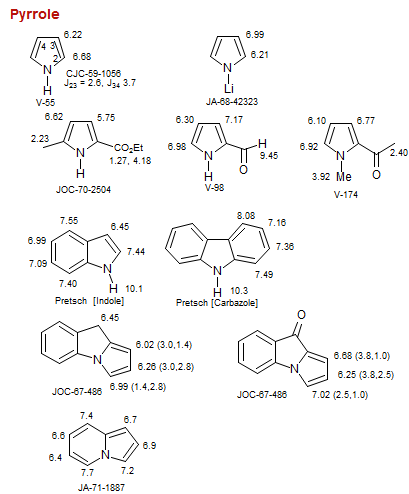
Pyrrole

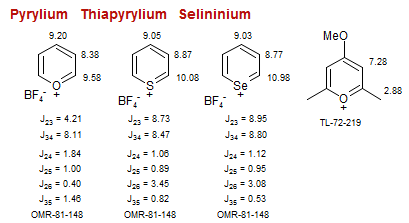
Pyrylium

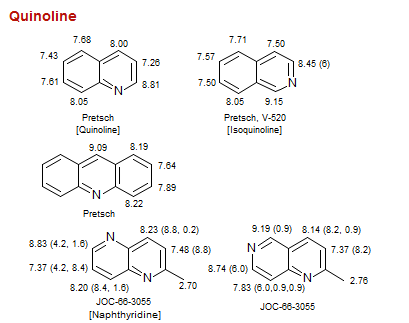
Quinoline

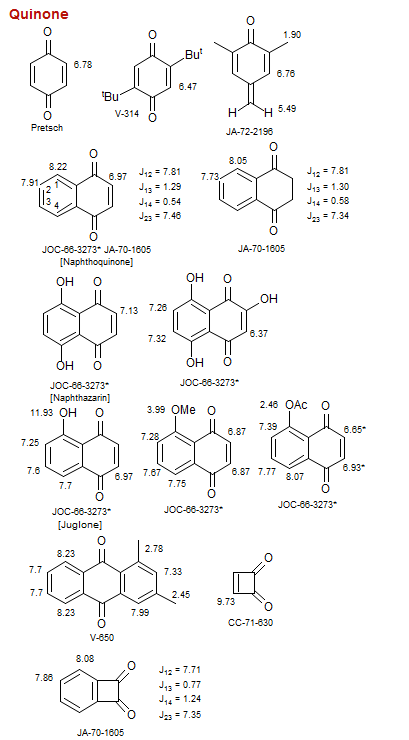
Quinone

Quinoxaline

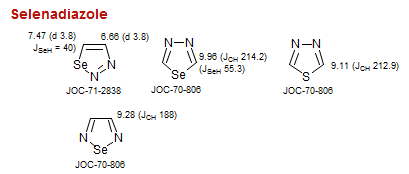
Selenadiazole

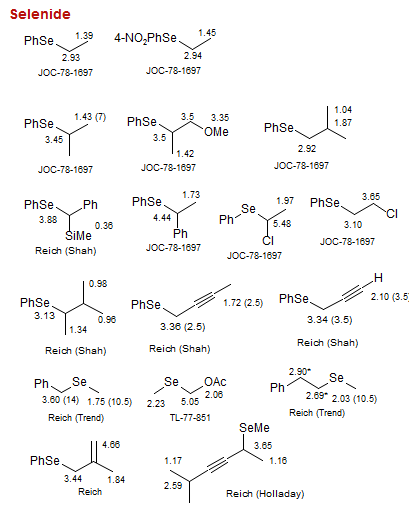
Selenide

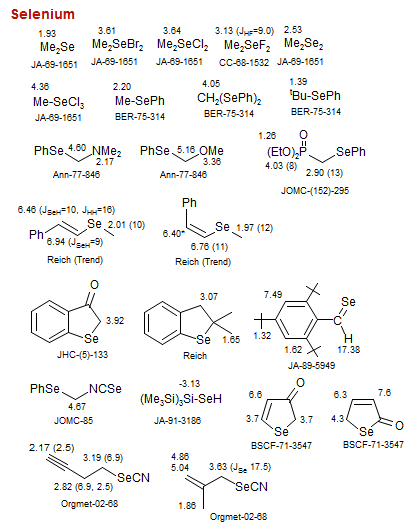
Selenium

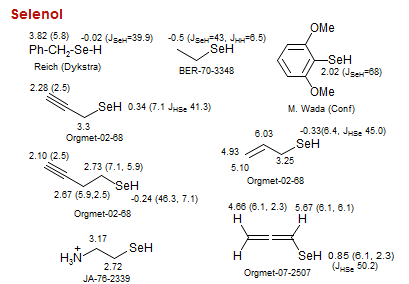
Selenol

Selenonium

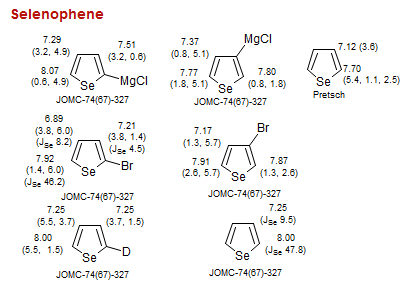
Selenophene

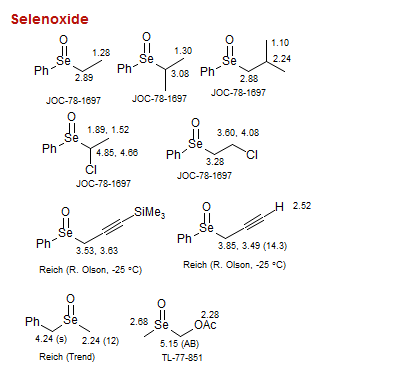
Selenoxide

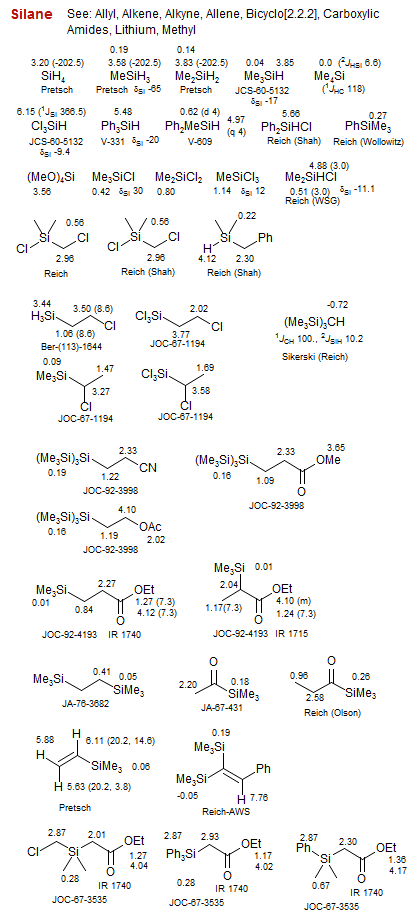
Silane

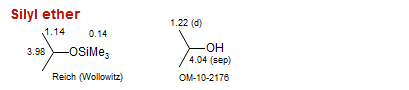
Silyl ether

Spiro

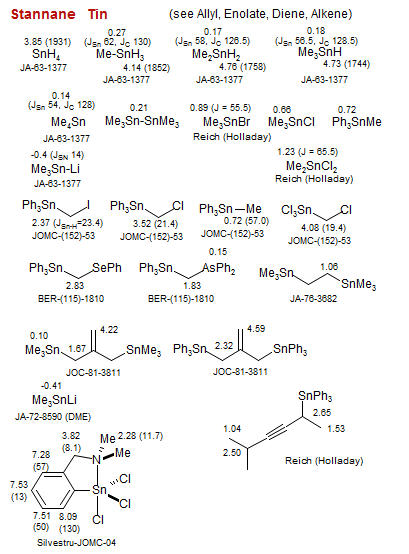
Stannane

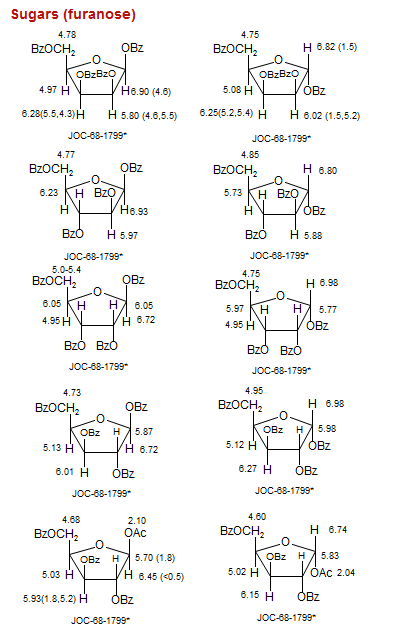
Sugars (furanose)

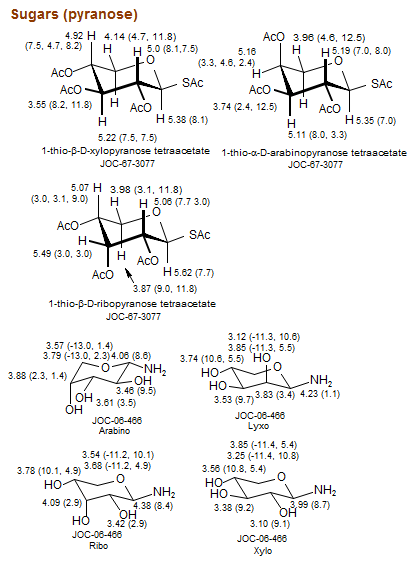
Sugars (pyranose)

Sulfates

Sulfenate

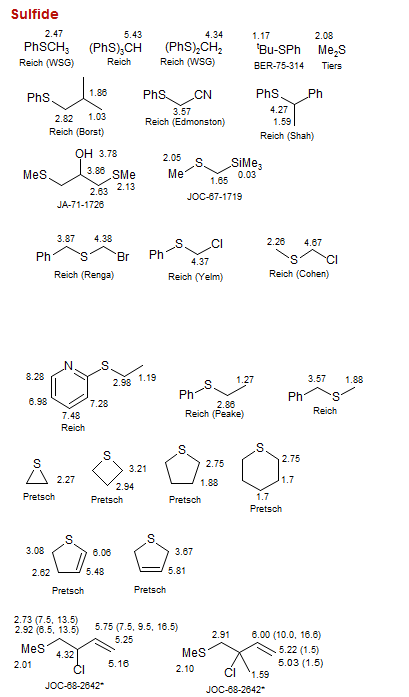
Sulfide

Sulfilimine

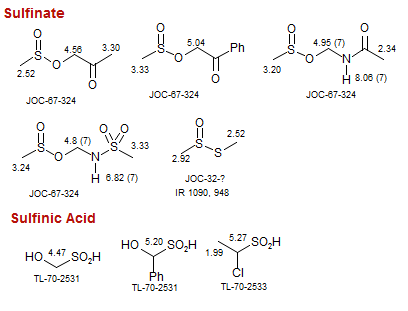
Sulfinate

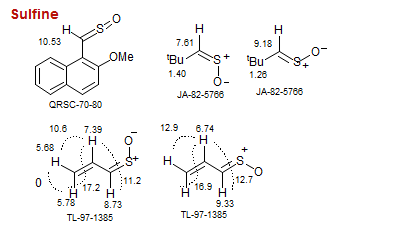
Sulfine

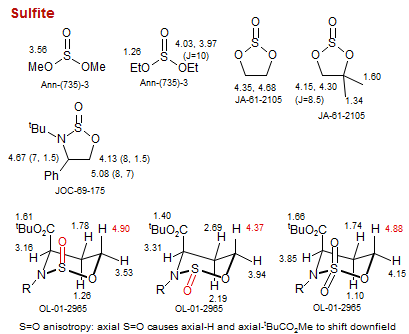
Sulfite

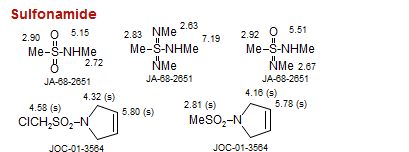
Sulfonamide

Sulfonate, thio

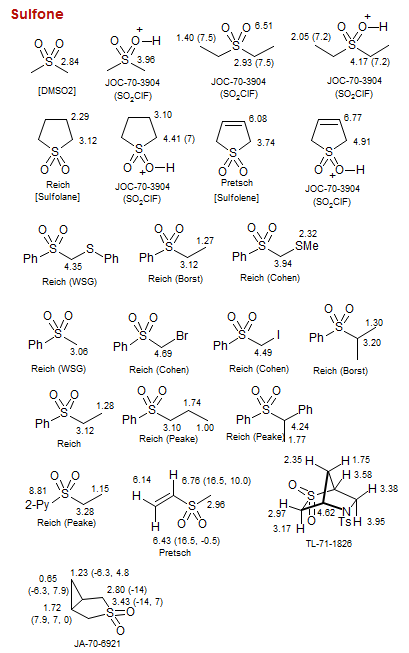
Sulfone

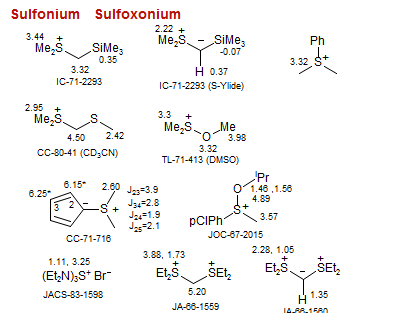
Sulfonium

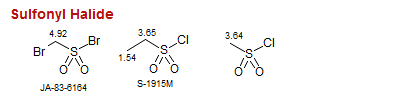
Sulfonyl Halide

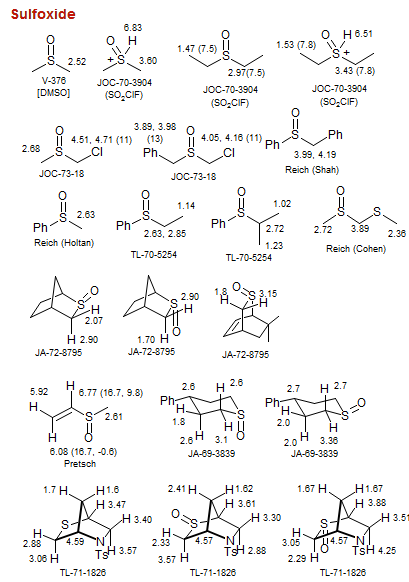
Sulfoxide

Sulfur

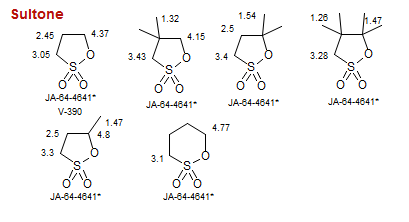
Sultone

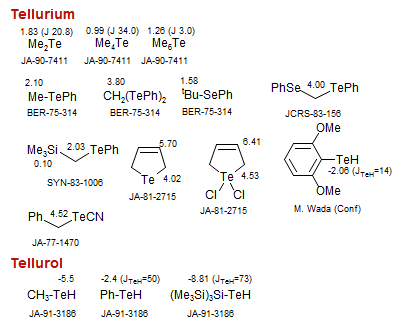
Tellurium

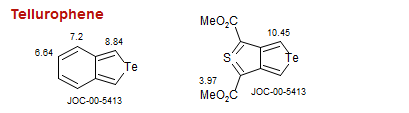
Tellurophene

Tetrahedrane

Tetrahydrofuran

Tetrazole

Thallium

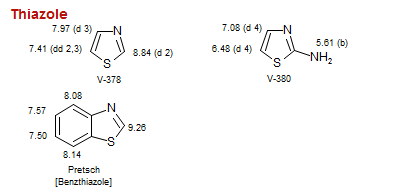
Thiazole

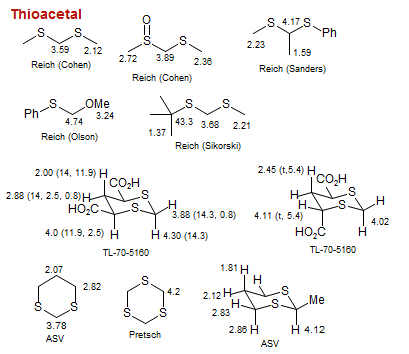
Thioacetal

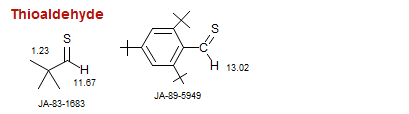
Thioaldehyde

Thioaldehyde S-oxide

Thioamide

Thiocarboxylic Acid

Thiocyanate

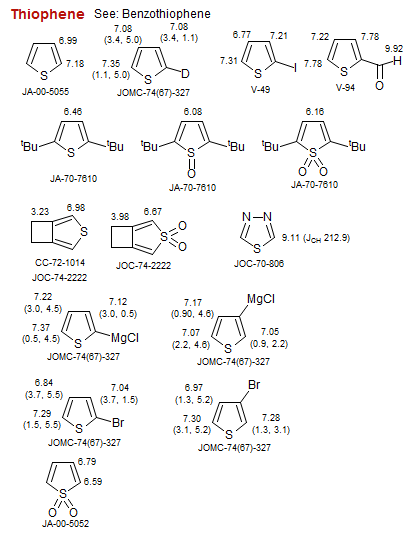
Thiophene

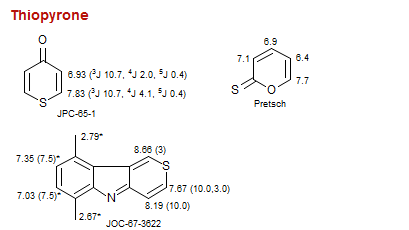
Thiopyrone

Triazine

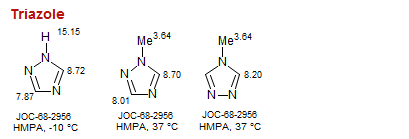
Triazole

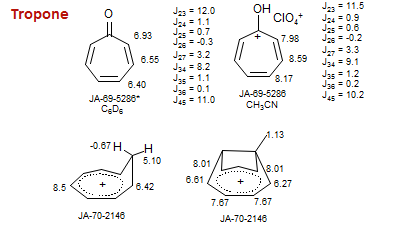
Tropone

Tungsten

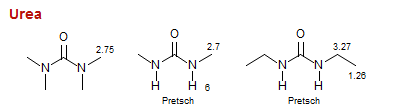
Urea

Water

Xylylene, ortho-

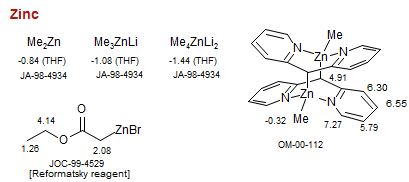
Zinc

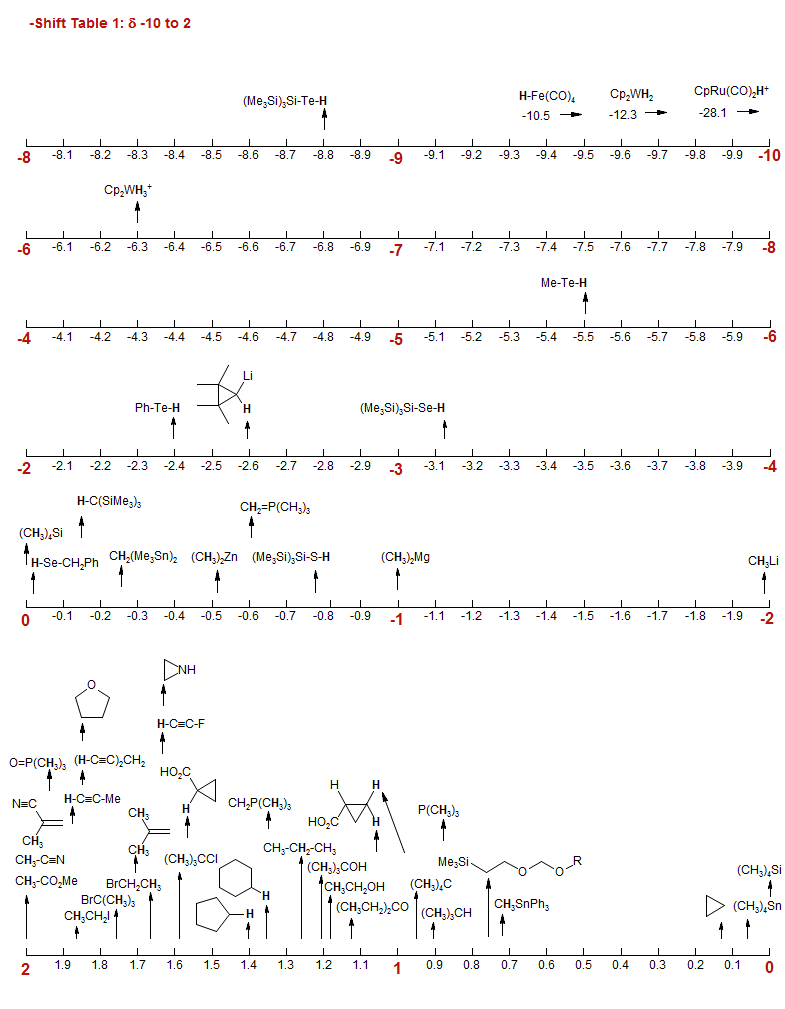
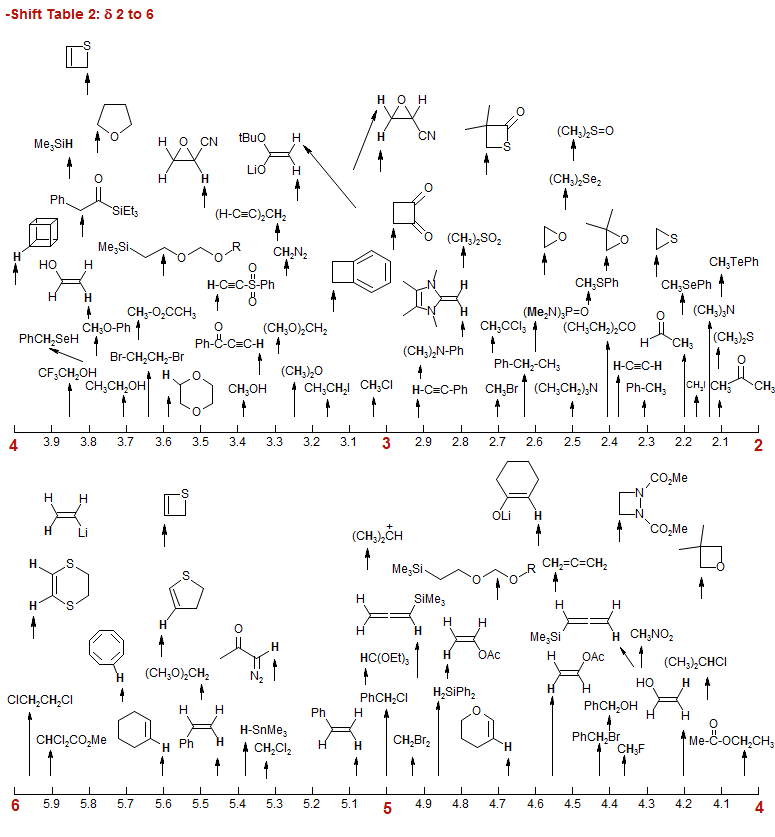
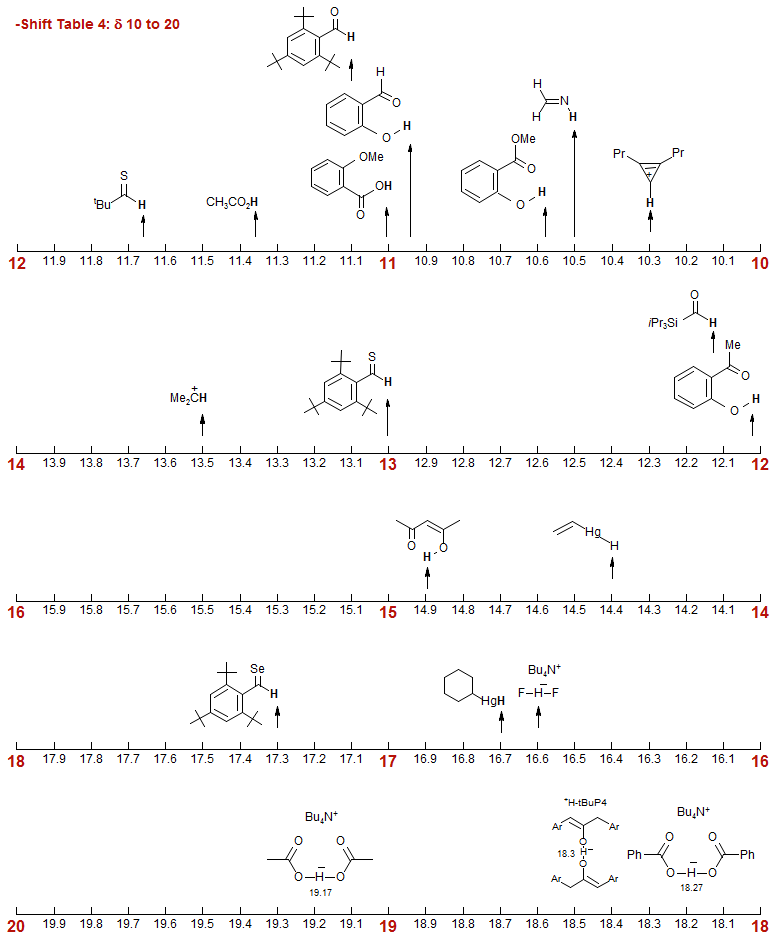

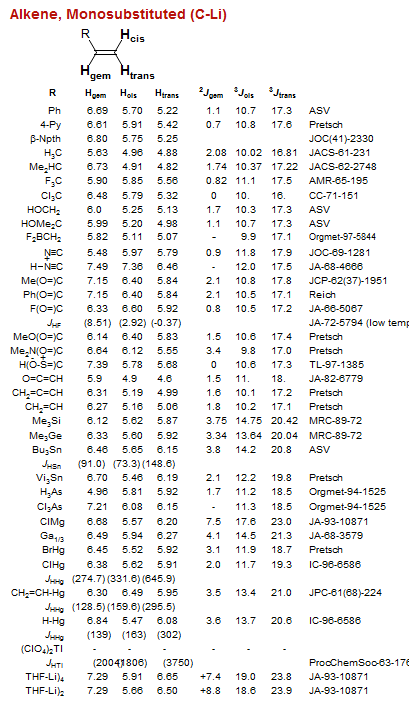
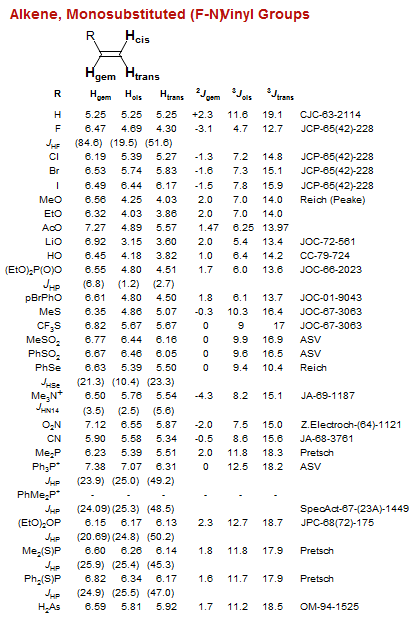
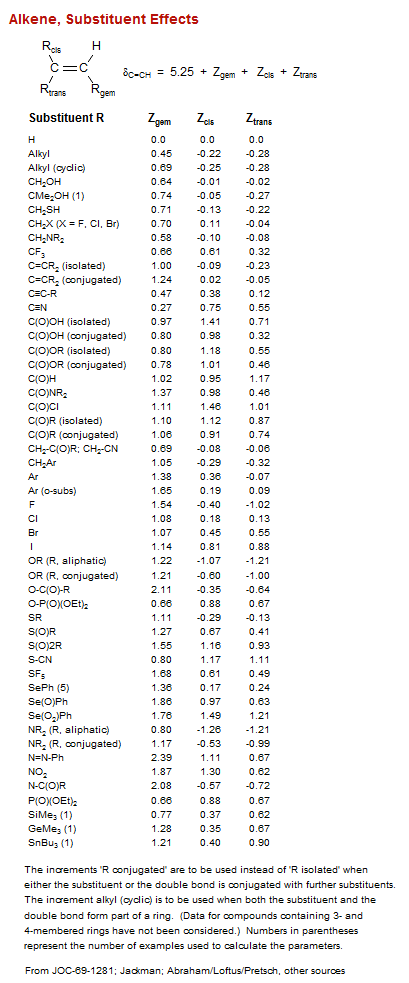
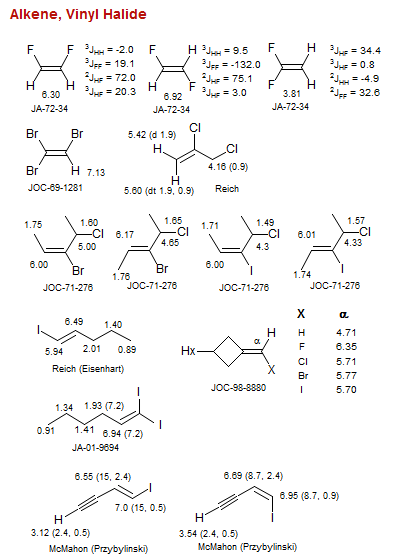
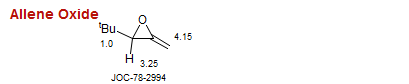
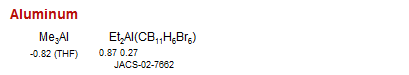

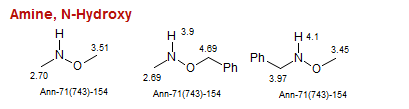

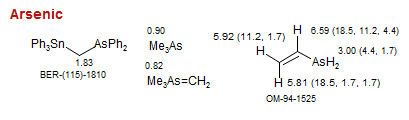
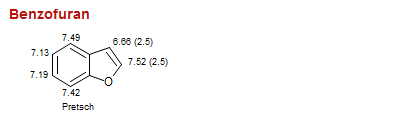

![Bicyclo[1.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata47.gif)
![Bicyclo[1.1.1]](/hansreich/resources/nmr/nmr_data/h_data/hdata48.gif)
![Bicyclo[2.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata55.gif)
![Bicyclo[2.1.1]](/hansreich/resources/nmr/nmr_data/h_data/hdata54.gif)
![Bicyclo[2.2.1]](/hansreich/resources/nmr/nmr_data/h_data/hdata52.gif)
![Bicyclo[2.2.2]](/hansreich/resources/nmr/nmr_data/h_data/hdata53.gif)
![Bicyclo[3.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata61.gif)
![Bicyclo[3.1.1]](/hansreich/resources/nmr/nmr_data/h_data/hdata60.gif)
![Bicyclo[3.3.1]](/hansreich/resources/nmr/nmr_data/h_data/hdata56.gif)
![Bicyclo[4.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata63.gif)
![Bicyclo[5.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata64.gif)
![Bicyclo[6.1.0]](/hansreich/resources/nmr/nmr_data/h_data/hdata65.gif)