5-HMR-4 Geminal Proton-Proton Couplings (2JH-H)
Two-bond H-H couplings (Review: Tet. 1969, 25, 4681) vary in a complicated way with structure, and they can only be understood if both magnitude and sign is taken into account. Some extreme examples are given below.
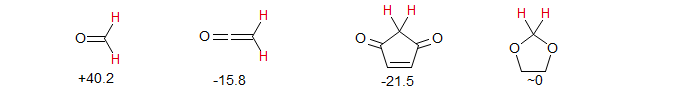
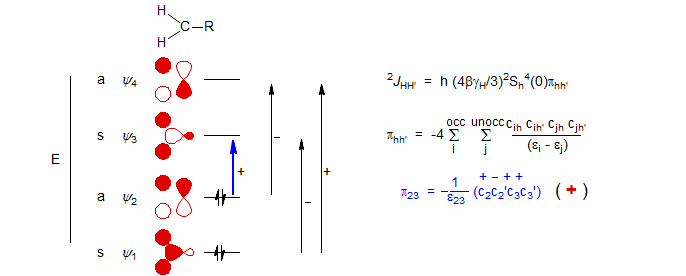
Most 2J couplings fall into two well-defined groups. For unstrained sp3 CH2 protons with innocuous substituents, the coupling is typically around -12 Hz, whereas the 2-bond coupling of sp2 (vinyl) protons is much smaller, typically 2 Hz. The molecular orbital perturbation theory of Pople and Bothner-By (J. Chem. Phys. 1965, 42, 1339) predicts the electronic effects of substituents on these coupling constants based on the interaction between filled and empty orbitals of the CH2 fragment. Excitation between orbitals of the same symmetry has a negative effect on J, between orbitals of opposite symmetry has a positive effect. The 1/E term is largest for the HOMO-LUMO transition, so coupling effects are dominated by the ψ2 ψ3 transition. Substituents which reduce the energy gap between ψ2 and ψ3 (i.e. raise ψ2 or lower ψ3) will increase the size of 1/E and thus have a (+) effect on the coupling, whereas those which increase the energy gap (i.e. lower ψ2 or raise ψ3) will have a (-) effect. There are also changes in the orbital coefficients which affect the magnitude of the coupling.
σ-Acceptor substituents (electronegative atoms like F, O, N) interact mainly with ψ3 and ψ1 because of symmetry restrictions. The most important effect is to lower ψ3, and thus have a (+) effect on the coupling, whereas σ-donor substituents like Si or other metals will raise ψ3 and thus have a (-) effect.
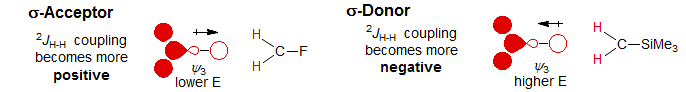
Remarkably, π-donors and acceptors have the opposite effect -- symmetry requires that these will interact mainly with ψ2. Thus π-donor substituents (directly attached atoms with lone pairs, or adjacent electron rich bonds) will raise ψ2, and result in a (+) effect, and π-acceptor substituents (carbonyl groups and related functions, or adjacent electron poor bonds) will lower ψ2 and have a (-) effect.
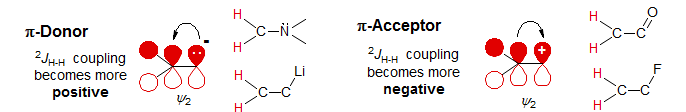
Gem coupling in Saturated Carbons (sp3): In acyclic and unstrained ring systems the gem coupling is typically from -10 to -13 Hz.
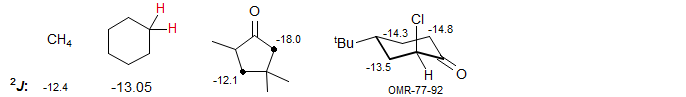
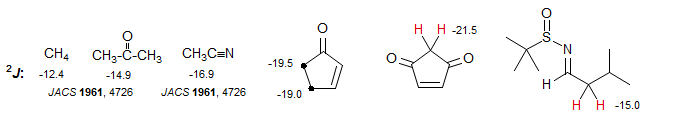
Substituents will change these couplings as described above: when the CH2 group is substituted with a π-acceptor like a carbonyl, imino or cyano group, the coupling becomes more negative, i.e. larger in magnitude, ranging from -15 to -25 Hz. This is a reliable and important effect which can help with structure assignments. Examples: 1, 2, 3, 4, 5, 6, 7, 8.
Conjugating aryl, alkene and alkyne substituents also make the coupling more negative (sp2 and sp carbons are more electronegative than sp3 carbons). An extreme example of an exceptionally large π-acceptor effect is shown by the bis-protonated binol-derived dication, which has the largest gem coupling at an sp3 carbon reported.

Substituents like the halogens, alkoxy and amino groups are both σ-acceptors and π-donors. Both are (+) effects, so the couplings become more positive (i.e. smaller numbers), in some cases they are close to zero. Examples: 1, 2, 3, 4, 5, 6, 7.

Ring strain has a (+) effect on gem coupling. Thus in cyclopropane the coupling has increased from -12 to -4 Hz. Examples: 1, 2. The additional (+) effects of oxygen bring the coupling to +2 in aziridine and +5.5 in ethylene oxide. Examples: 1, 2, 3, 4, 5, 6.

Gem coupling in Unsaturated Carbons (sp2): The gem coupling in ethylene itself is +2.5 Hz, and most terminal alkenes have small couplings in the range of 1-3 Hz. Electronegative substituents (F, O) on the double bond behave as π-acceptors, with a (-) effect on the coupling, which can become close to zero for weakly accepting substituents (as in methyl vinyl sulfide). Electropositive substituents on the neighboring carbon (Si, Li) behave as π-donors with a (+) effect on the coupling. For α-trimethylsilylvinyllithium both substituents have a (+) effect, and result in an exceptionally large coupling, whereas in α-ethoxyvinyllithium the two substituents have opposite effects, and the coupling was too small to observe. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13.

The large positive coupling in formaldehyde, and large negative coupling in ketene can be understood in these terms as well. For formaldehyde the oxygen substituent behaves as a strong σ-acceptor as well as a strong π-donor from the π-lone pair, both (+) effects, rendered especially large because of the short bond distance. Imines also show large positive 2J.

In a similar vein, for ketene the carbonyl substituent behaves as a strong π-acceptor, giving an usually large negative coupling. In allenes, the sp2-carbon "substituent" behaves as a weak electron acceptor (sp2 carbons are more electronegative than sp3 carbons), also leading to relatively large negative 2J values. Examples: 1, 2.

Geminal Proton-Proton Couplings Summary (2JH-H)
Geminal couplings between protons vary widely in sign and magnitude. There are both positive and negative substituent effects on the coupling, as summarized below. The remarkable feature is that σ and π acceptors have opposite effects on the coupling, as do σ and π donors.

Exercise: Identify the two multiplets below, get δ and J, and assign them to protons in the structure. Explain your assignment.

Next Section: Three-bond Coupling · Previous Section: Spin-Spin Splitting · Home