5-HMR-7 Pople Nomenclature for Coupled Spin Systems
The analysis of complex NMR patterns is assisted by a general labelling method for spin systems introduced by Pople. Each set of chemically equivalent protons (or other nuclei) is designated by a letter of the alphabet. Nuclei are labeled AX or AMX if their chemical shift differences are large compared to the coupling between them (Δδ > 5J). Nuclei are labeled with adjacent letters of the alphabet (AB, ABC, MN or XYZ) if they are close in chemical shift compared to the coupling between them (i.e. if they are strongly coupled).
If groups of nuclei are magnetically equivalent, they are labeled AnBn, etc. Thus CH3 groups are A3, or X3. A group of magnetically equivalent nuclei must have identical chemical shifts, and all members of the group must be coupled equally to nuclei outside the group. If nuclei are chemical shift equivalent but not magnetically equivalent, then they are labeled AA', BB'B'' or XX'. Thus in an A2B2 system the A nucleus must have identical couplings to the two B nuclei. In an AA'BB' system, on the other hand, JAB ≠ JAB'. There are usually profound differences in the appearance of A2B2 compared to AA'BB' patterns.
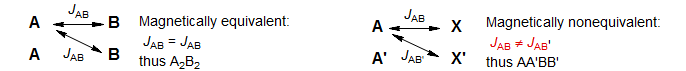
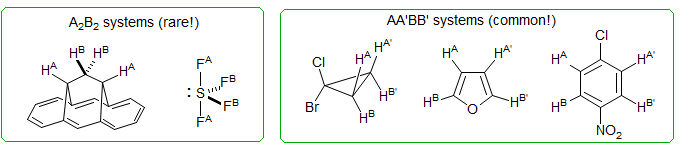
Here some examples to illustrate the difference between A2B2 and AA'BB'
5-HMR-7.1 Two-Spin Systems
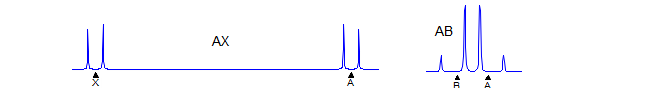
AX
First order. Significant parameters: JAX. A and X are each doublets. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10.
J is directly measurable, νA and νB must be calculated. Intensities are distorted: the doublets are not 1:1; the inner lines are larger, the outer lines smaller. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. see NMR Gallery AB Spin system
5-HMR-7.2 Three-Spin Systems
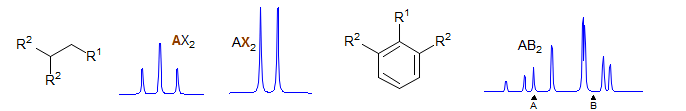
AX2
First order. Significant parameters: JAX. A is a triplet, X is a doublet. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
Second order. Both JAB and νAB must be calculated - neither can be directly measured from the spectrum. Significant parameters: JAB, νAB. Examples: 1, 2, 3.
AMX
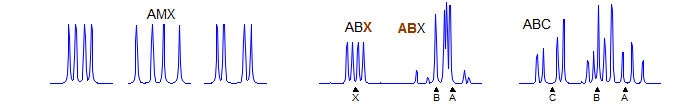
First order. Significant parameters: JAM, JAX, JMX. A, M and X are each doublet of doublets (assuming all three couplings are large enough to detect). Typical systems are vinyl groups, trisubstituted benzenes, disubstituted pyridines, and monosubstituted furans and thiophenes. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18.
Second order. This is a very common pattern. JAB is directly measurable. The parameters JAX, JBX, νA and νB can be calculated from the line positions of the spectrum once it has been properly analyzed. Examples: 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. see NMR Gallery ABX Spin system
ABC
Second order. This pattern can only be accurately solved using computer simulation methods. Manual analysis as a distorted ABX or even AMX pattern will lead to approximate values of coupling constants, which in severe cases can be drastically wrong. Examples: 1
5-HMR-7.3 Four-Spin Systems
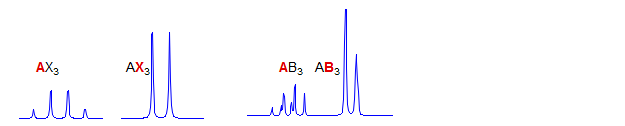
AX3
First order. Significant parameters: JAX. Commonly seen in CH3CHXY groups. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17.
AB3
Second order. Computer simulation required to solve.
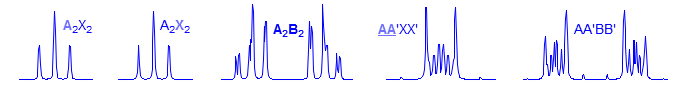
A2X2
First order. This is a very rare pattern. A and X are each triplets.
Second Order. Rare.
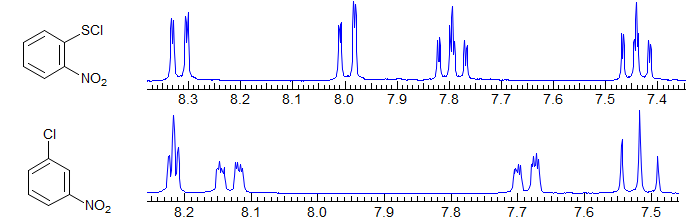
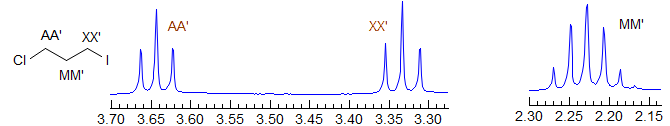
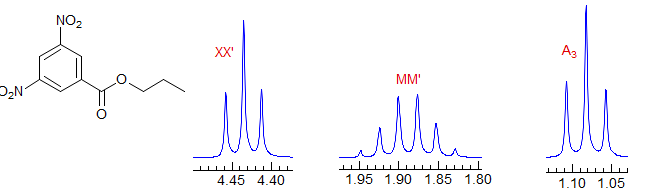
Second order. Common pattern. Can be solved by hand, but there are several ambiguities. For example, one cannot distinguish JAA' from JXX'. Significant parameters: JAA', JXX', JAX, JAX'. The AA' and XX' patterns are each centrosymmetric, and they are identical to each other, hence the inability to distinguish A from X parameters. A common type is X-CH2-CH2-Y, which is often just two triplets. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9. Also common are p-disubstituted benzenes. Examples: 1, 2, 3, 4, 5, 6.
AA'BB'
Second order. This is a common pattern. The entire multiplet is centrosymmetric (i.e., the AA' part is a mirror image of the BB' part). Requires computer simulation to solve. Seen in X-CH2CH2-Y groups where X and Y have similar shift effects, (Examples: 1, 2, 3, 4) in 2,2-disubstituted dioxolanes,(Examples: 1, 2), in 1,2-disubstituted cyclopropanes (Examples: 1, 2) in symmetrical 1,2-disubstituted aromatics (Examples: 1, 2) and in many other types of symmetrical structures.
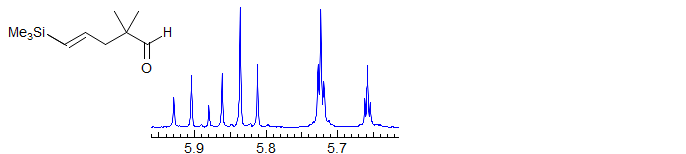
ABX2
Seen in CH=CH-CH2 and CH-CH-CH2 part structures in achiral molecules.(Examples: 1, 2, 3, 4, 5, 6, 7.
ABMX
First or second order depending on νAB and νMX. Structural types include -CH2-CH2-, -CH-CH2-CH- and -CH2-CH-CH- in chiral molecules where the CH2 groups are diastereotopic. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26.
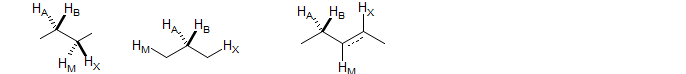
Also commonly seen in 1,4-disubstituted dienes as well as in 1,2- and 1,3-disubstituted benzenes, 2- or 3-substituted pyridines and related aromatics. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.
5-HMR-7.4 Five-Spin Systems
A2X3
First order. Very common pattern: ethyl groups: CH3CH2-R where R is an achiral electron withdrawing group (if R is chiral then we get an ABX3 pattern) Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 15.
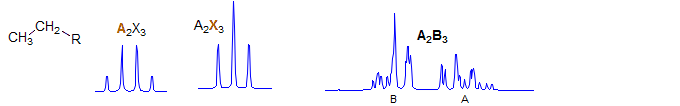
A2B3
Second order. Seen in ethyl groups CH3CH2-R where R is a metal: e.g. CH3CH2-SiR3. Examples: 1, 2, 3, 4.
Second order, but soluble by hand. Commonly seen in ethyl groups in chiral molecules where the CH2 protons are diastereotopic. For these the X part is always a triplet. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. Methyl-substituted alkenes (CH=CH-CH3) and related alkane systems (CH-CH-CH3) can also give this pattern, but here the methyl group is always a dd or d. Examples: 1, 2, 3, 4, 5, 6.
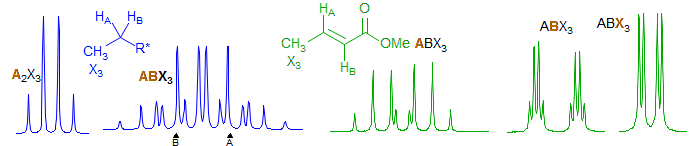
AA'MM'X
Second order. Part structures like -CH2-CH2-CH- can sometimes appear to be first order in acyclic structures (t, dt, t), but they are more complex in cyclic compounds . Examples: 1, 2, 3, 4, 5.
AA'BB'C
Always second order. Commonly seen in monosubstituted phenyl groups. Examples: 1, 2, 3, 4.
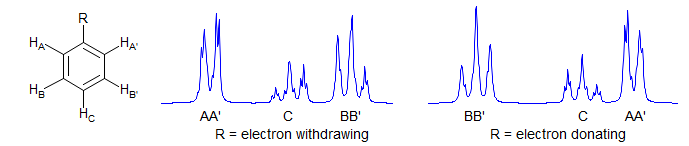
Monosubstituted cyclopropanes are also AA'BB'C systems. Example: 1
A2MXY
A common type involves isolated allyl groups in achiral molecules. Examples: 1, 2, 3, 4, 5, 6, 7.
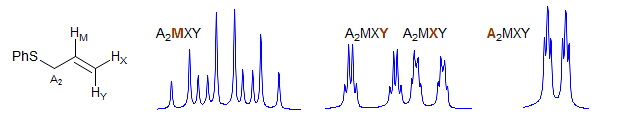
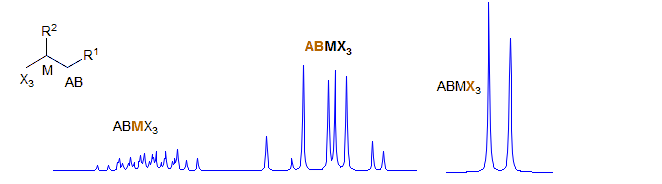
ABMXY
Part structures like R1-CH2-CH-CH2-R3 are actually two ABX patterns which share a common X. If νAB and νXY are large enough they can be close to first order. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. If R1 and R3 are the same, then the pattern is technically AA'BB'X but shows no unusual complexity because there is no significant coupling between the A and A' protons, nor between the B and B' protons. Such systems are better defined as (AB)2X to indicate that magnetic inequivalence is not a significant factor. Examples: 1, 2, 3, 4, 5, 6. This spin system also appears in HC=CH-CH-CH2.Examples: 1.
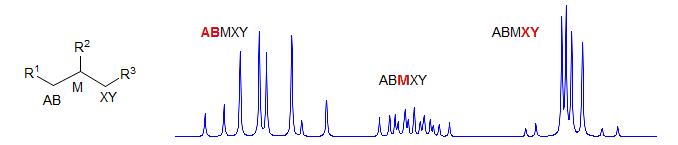
ABMNX
Seen in systems like -CH2-CH2-CH in chiral molecules. Can be first order if all the shifts are well separated, but gets very complicated if any of the coupled protons are superimposed. R2 and R3 must be different or one of the R groups must be chiral to get AB MN instead of AA' MM' patterns. Examples: 1, 2, 3, 4, 5, 6.

5-HMR-7.5 Six-Spin Systems
AA'MM'XX'
Second order, but a common type, R1-CH2-CH2-CH2-R2 (R1 and R2 achiral) often looks nearly first-order, especially if R1 and R2 are relatively small groups. Usually two apparent triplets and a pentet. Examples: 1, 2, 3, 4, 5, 6, 7, 8. If R1 and R2 are identical a pentet (2H) and a triplet (4H) is often seen. Examples: 1, 2.
ABMNXY
If R1 and/or R2 are chiral, then R1-CH2-CH2-CH2-R2 form a complex series of multiplets since all protons are chemically shifted. Examples: 1, 2. This spin system also appears in structures like R1-CH2-CH-CH-CH2-R2 and R1-CH-CH-CH2-CH2-R2. Examples: 1, 2, 3, 4, 5.
Common types are 1,1- or 1,2-disubstituted propyl groups. For the former, if R1 and R2 are different, the AB part is an AB quartet, each half of which is split by three protons, thus an AB quartet of pentets, or an AB quartet of doublets of doublets. Examples: 1, 2, 3, 4, 5, 6, 7, 8. If R1 and R2 are the same, or the chemical shift between A and B is very small, then the AB part might be a pentet or a doublet of quartets. Examples: 1, 2.

If R1 and R2 are vicinal then the AB part always has the appearance of the AB part of an ABX system, the M part is ddq, and the X3 part a doublet. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10.
5-HMR-7.6 Seven-Spin Systems
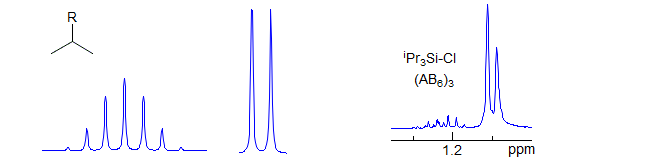
AX6
Common pattern: isopropyl groups in achiral molecules: (CH3)2CH-R where R is a heteroatom or a carbon bearing no protons (in chiral molecules, the CH3 groups will be diastereotopic, hence an A3B3X system). Technically, these are A3A'3X systems, but they are first order because there is no coupling betwen A and A'. Usually a septet and a doublet. Examples: 1, 2, 3, 4, 5, 6, 7. When R is a metal like Si or Sn the CH3 and CH protons can be close in chemical shift, and give a complex pattern (AB6) or even a singlet.
A3MM'XX'
Not actually first order. However, a common type, n-propyl groups CH3-CH2-CH2-R, often appear to be nearly first order if chemical shifts are large enough, thus approximately two triplets and a sextet. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.
Next Section: Symmetry · Previous Section: Long-range Coupling · Home