5-HMR-8 Symmetry in NMR Spectra
The symmetry properties of molecules have profound effects on NMR spectra, and, in appropriately symmetric structures, can provide valuable structural information distinct from that provided by chemical shifts and coupling. Examples of structure assignments using symmetry properties: 1, 2, 3, 4.
Protons and other nuclei in NMR spectra can be classified as heterotopic, diastereotopic, enantiotopic and homotopic. Heterotopic and diastereotopic protons will have different chemical shifts and couplings to neighboring magnetic nuclei, enantiotopic and homotopic protons will have identical chemical shifts. They may or may not have identical couplings to other nuclei. Distinction can be made by the substitution test.
5-HMR-8.1 The Substitution Test for Equivalence of Protons
For a pair of protons to be tested, replace one and then the other with another group (one not present in the molecule). Compare the two structures formed. If they are identical, the protons are homotopic, if they are enantiomers, the protons are enantiotopic, if they are diastereomers then the protons are diastereotopic, if they are structural isomers, the protons are constitutionally heterotopic.
Homotopic Protons:
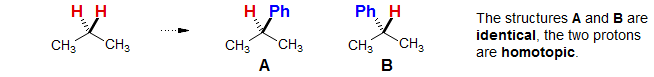
Enantiotopic Protons:
Enantiotopic protons normally have identical chemical shifts. However, when the molecule is placed in a chiral environment (say with an optically active solvent, co-solvent or Lewis acid) then the protons can become diastereotopic. This is in contrast to homotopic protons, which are always identical.
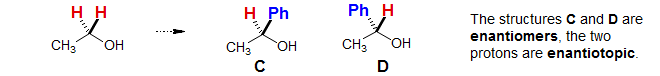
Diastereotopic Protons:
The concept of diastereotopicity was first introduced during the early days of NMR spectroscopy, when certain kinds of molecules gave unexpectedly complex NMR spectra, leading to some confusion about the origins of this hitherto undetected phenomenon (Nair, P. M.; Roberts, J. D. J. Am. Chem. Soc., 1957, 79, 4565). A typical situation where diastereotopic protons are seen is a CH2 group in a chiral molecule (one with an asymmetric center, or other types of asymmetry). See the sections on ABX, ABX3 and ABXYZ patterns for many additional examples of diastereotopic protons. For other illustrations of diastereotopic proton effects see: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. Carbons and other nuclei: 1 (C), 2 (C), 3 (Se)
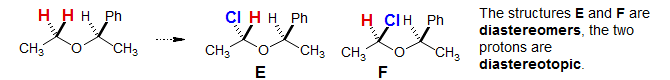
A more subtle form of diastereotopism is demonstrated in the classical example of diethyl acetal below. Even though diethyl acetal has no asymmetric centers, each CH2 group is diastereotopic. This can be shown by applying the substitution test, which creates a pair of diastereomers G and H. Thus the ethyl group forms an ABX3 pattern (see Section 5-HMR-13). The key to understanding this type of diastereotopicity is that the molecule has a plane of symmetry (hence is achiral). However, there is no plane of symmetry that bisects the CH2 protons, so they are nonequivalent. For additional examples of these types of diastereotopic groups see: 1, 2, 3, 4, 5, 6, 7, 8, 9.
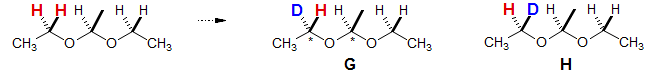
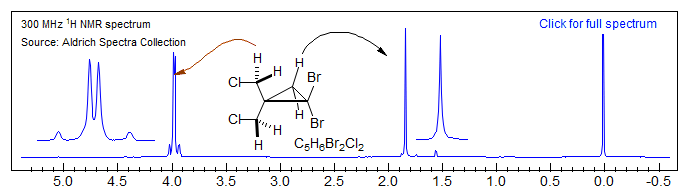
The dibromocyclopropane spectrum illustrates this effect in a different context - the protons of the CH2Cl group are diastereotopic. However, the protons of the cyclopropane CH2 group are not, since they are related by a plane of symmetry.
Not all CH2 groups in chiral molecules are diastereotopic - in the chiral molecules below the CH2 is on a C2 axis of symmetry, and the protons are homotopic. In general, CH2 groups (or other similar groups like CHMe2, CHF2, etc) will be diastereotopic when part of a chiral molecules unless the CH2 group is on a C2 rotation axis.
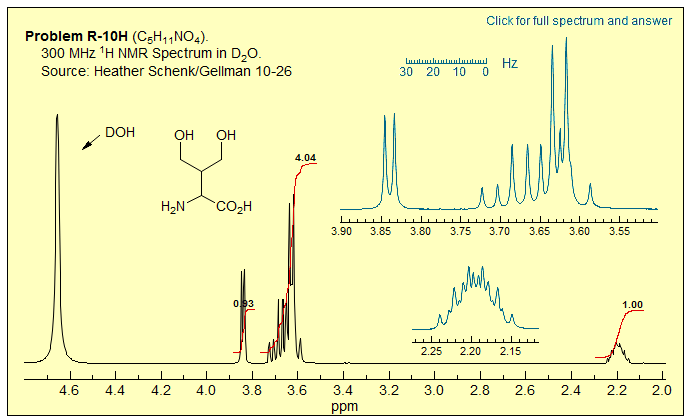
Exercise: A graduate student thought she had prepared the compound below, but was worried about the NMR spectrum (taken in D2O), which seemed more than a little odd. Does the NMR spectrum fit the structure? Analyze and assign each of the multiplets. In particular, provide an explanation for the appearance of the key multiplet δ 3.5-3.8, get all δ and J. This is a particularly devious example of diastereotopic effects. Click spectrum for answer.
Exercise: Why are three of the aromatic 13C NMR signals in this compound doubled?
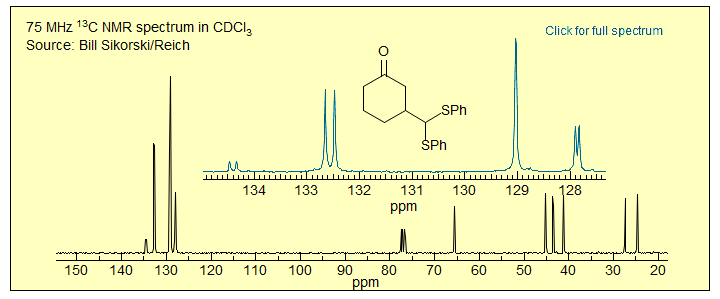
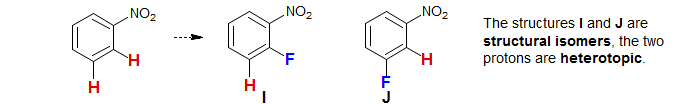
Constitutionally Heterotopic Protons:
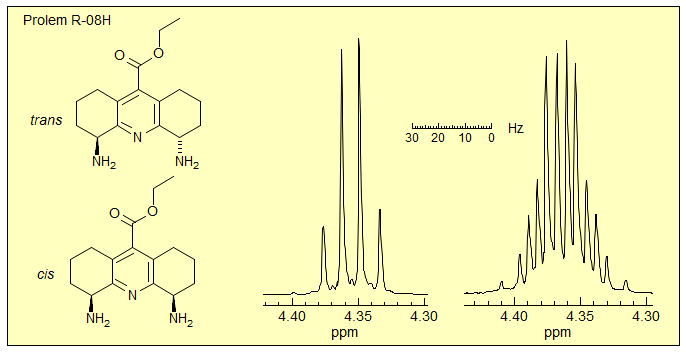
Exercise: Partial 500 MHz 1H NMR spectrum of two stereoisomers of a pyridyl diamine are shown below. Each signal integrates to 2 protons. These spectra were used by the researchers to assign stereochemistry. (Kneeland, D. M.; Ariga, K.; Lynch, V. M.; Huang, C.-Y.; Anslyn, E. V. J. Am. Chem. Soc. 1993, 115, 10042) Assign the signals, explain why they are different, and assign the structures. HINT: review ABX3. Click spectrum for answer
5-HMR-8.2 Magnetic Equivalence
There is an additional element of symmetry which is important for NMR spectra that involve J-coupling, the magnetic equivalence or inequivalence of nuclei. Protons that are enantiotopic or homotopic will have the same chemical shift, but they will not necessarily be magnetically equivalent. For two protons to be magnetically equivalent they not only have to have the same chemical shift, but they must also each have the same J coupling to other magnetic nuclei in the molecule. This is easiest to see from some specific examples.
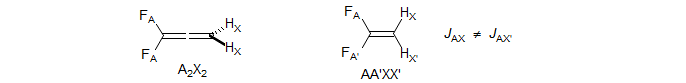
The two vinyl and two allylic protons in cyclopropene are each magnetically equivalent because each of the A protons is equally coupled to the two X protons. The spectrum consists of two identical triplets (A2X2 system).
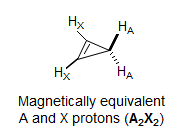
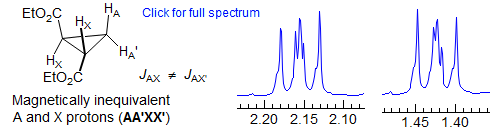
On the other hand, the two pairs of equivalent protons in trans-bis(carboethoxy)cyclopropane are NOT magnetically equivalent, because each of the A protons is coupled differently to the two X protons (one is a trans coupling, the other a cis). In the Pople nomenclature, such magnetically inequivalent nuclei are given an AA' designation. Thus the bis(carbomethoxy)cyclopropane is referred to as an AA'XX' system, where A and A' refer to protons that are symmetry equivalent but not magnetically equivalent. The spectrum will be much more complicated than two triplets, and both sets of proton signals will be identical.
Two more examples are 1,1-difluoroallene, which is an A2X2 system, and 1,1-difluoroethylene, which is an AA'XX' system (see 5-HMR-15 for a spectrum).
In general any system which contains chemical shift equivalent but magnetically inequivalent nuclei of the AA' type will not give first order splitting patterns, although sometimes the spectra may appear to be first order ("deceptively simple" spectra). For example, X-CH2-CH2-Y systems are of the AA'XX' type, but the coupling constants JAX and JAX' are often close enough in size that apparent triplets are seen for each CH2 group. See Section 5-HMR-15 for examples.
Two important generalizations:
The NMR Time Scale
It is important to recognize that diastereotopic and magnetic equivalence effects are subject to the time scale of the NMR experiment, which is on the order of tenths of a second (see Sect 8-TECH-3). Flexible molecules will often have several conformations, some of which may have lower symmetry than others. However, since these conformations are typically interconverting rapidly on the NMR time scale, the observed symmetry in the NMR spectrum will be that of the most symmetric conformation reachable. Thus cyclohexane is a sharp singlet at room temperature, whereas at -100 °C the ring inversion is slow on the NMR time scale, and a much more complex spectrum results (see Sect 5-HMR-5.3).
Next Section: Second Order · Previous Section: Pople Spin Nomenclature · Home