5-HMR-11 The AX2 and AB2 Patterns
Some types of molecules containing three spin systems in which two of the nuclei are magnetically equivalent are shown below. Perhaps the most common are 1,2,3-trisubstituted benzenes in which the 1 and 3 substituents are identical, or 1,3,5-trisubstituted benzenes in which two substituents are identical. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, ?. see NMR Gallery AB2 Spin system
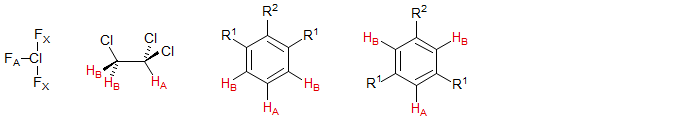
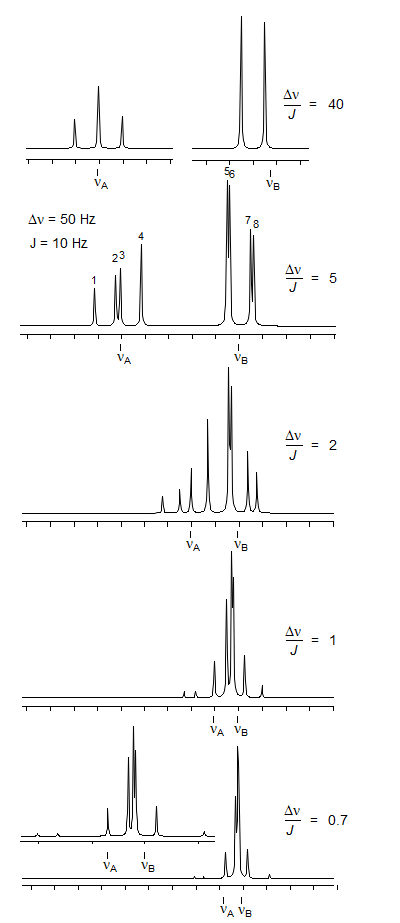
AX2 and AB2 patterns are not as common as AMX, ABX and ABC, but there is a point to examining them in some detail. The transition of an AX2 to an AB2 spin system provides additional insight into the appearance of second order effects. In the AB pattern there are two effects of this type:
• The line intensities no longer follow simple rules
• The arithmetic average of line positions no longer gives true chemical shifts, although the coupling constant JAB can still be directly measured from the spectrum.
In AB2 spectra a third and fourth effects appear: none of the line separations correspond to JAB, and additional lines appear which are not predicted by simple multiplet rules. The additional lines arise from splitting of double-intensity lines, as well as from the appearance of new transitions.
The AB2 spectra illustrate this process. In the top spectrum we have Δν/J >> 5, and the system is effectively AX2, it consists of an A triplet and a B doublet. As Δν/J becomes smaller, the double-intensity middle line of A and both B lines split into two lines. An additional line which is not a direct descendant of any of the AX2 lines appears (a combination line: αβα → βαβ). Since it is essentially a forbidden transition it is usually quite weak, but can sometimes be observed. In the bottom spectrum (Δν/J = 0.7) the intensity of line 9 is 0.4% of the most intense line (line 5).
When Δν/J < 1 the spectrum takes on the appearance of a triplet, with a very intense and broadened central line. Finally, as Δν/J approached 0 (νA = νB) the outer lines disappear completely, and we are left with a singlet.
Solving an AB2 Pattern
To analyze an AB2 pattern, we number the lines as shown, the four A lines ν1- ν4, the four B lines ν5- ν8, and the very weak combination line ν9. The arithmetic is simple:

Some points to remember about AB2 patterns:
1. The spectrum depends only on the ratio Δν/J.
2. Note that lines 1-4 must correspond to the one-proton part, lines 5-8 to the two-proton part. Thus, if the pattern is A2B then the numbering proceeds in the reverse direction. Distinguish the one and two proton parts by integration.
3. Line ν5 is the most intense line. The lines ν5 and ν6 often do not split up.
4. When Δν/J is much less than 1 the spectra appear nearly symmetrical since ν1, ν2 and ν8 become very weak. The spectrum then has the appearance of a distorted triplet with a 1:10:1 area ratio (the peak heights will not be in this ratio since the center line consists of several closely spaced ones.
5. Neither JBB nor the sign of JAB affect the appearance of the spectrum.

The "1H" part is upfield of the "2H" part here so numbers run from right to left: ν1 = 0.0 . . . ν8 = 40.7
νB = ν3 = 9.0 Hz (δB = (9 + 400)/60 = 6.82)
νA = (ν5 + ν7)/2 = (31.9 + 39.1)/2 = 35.5 Hz (δA = (35.5 + 400)/60 = 7.26)
|JAB| = |(ν1 - ν4 + ν6 - ν8)/3| = |(0 - 15.6 + 32.5 - 40.7)| / 3 = 7.9 Hz
Exercise: Assign the protons in 2-nitropyrene. There is some overlap. so consider separately how the protons in the parts of the molecule (a), (b) and (c) should look. Click spectrum for answer.

Next Section: ABX Patterns · Previous Section: AB and AX Patterns · Home