5-HMR-13 ABX3 Patterns
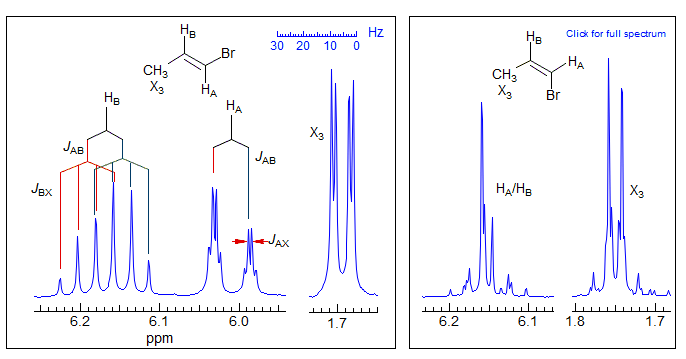
ABX3 patterns are very common in organic molecules. 1-Substituted propenes show this pattern. As can be seen in the example of trans-1-bromopropene below, the vinyl protons are essentially an AB quartet, each line of which is split into a quartet by the 2J and 3J coupling to the methyl groups. As is common for most 4-spin systems, when the chemical shift between the two of the protons becomes small (as in cis-1-bromo-1-propene) the spectrum becomes complicated, and can no longer be analyzed so simply. Examples: 1, 2, 3.
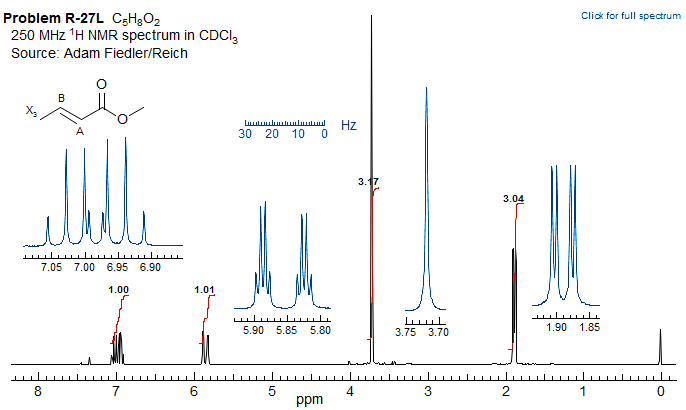
Another sample ABX3 spectrum of methyl crotonate is shown below (this one is really AMX3).
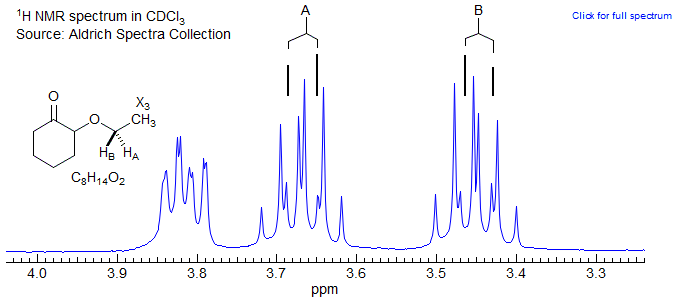
Most ethyl groups in chiral molecule will have diastereotopic CH2 protons, and thus form an ABX3 system. The partial 1H NMR spectrum of 2-ethoxycyclohexanone below illustrates a typical pattern. The A and B signals are well separated, and can be readily understood and solved as a first order "AMX3" pattern (each proton a leaning dq). Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13.
Just as for ABX systems, there is an exact solution, in which one first solves the four AB quartets, which are present in a 1:3:3:1 ratio (i.e., they represent the subspectra resulting from the four combinations of X spins: ααα; ααβ/αβα/βαα; αββ/βαβ/ββα; βββ). The solutions to these AB quartets give a 1:3:3:1 quartet for the A proton, and another for the B. These can then be solved as first order patterns.
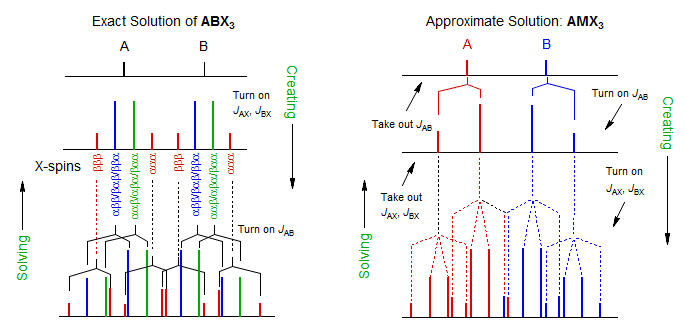
Fortunately, it is rarely necessary to do an exact solution. If JAX = JBX (or very nearly so), which is usually the case for CH3-CH2-R* systems, then a first order treatment of the pattern as an "AMX3" type is quite accurate. What is done here is to treat the pattern as an AB quartet, each line of which is split by the X3 protons into a 1:3:3:1 quartet. The four quartets will have the normal intensity ratios of an AB quartet. To solve, identify the AB-quartet of q and then remove the X coupling. What remains is an AB quartet which can be solved in the usual way. Note that this corresponds exactly to the "AMX" solution for ABX patterns (see 5-HMR-12.3), in which we treat the pattern as an AB quartet, each half of which is split into a doublet by the X nucleus.
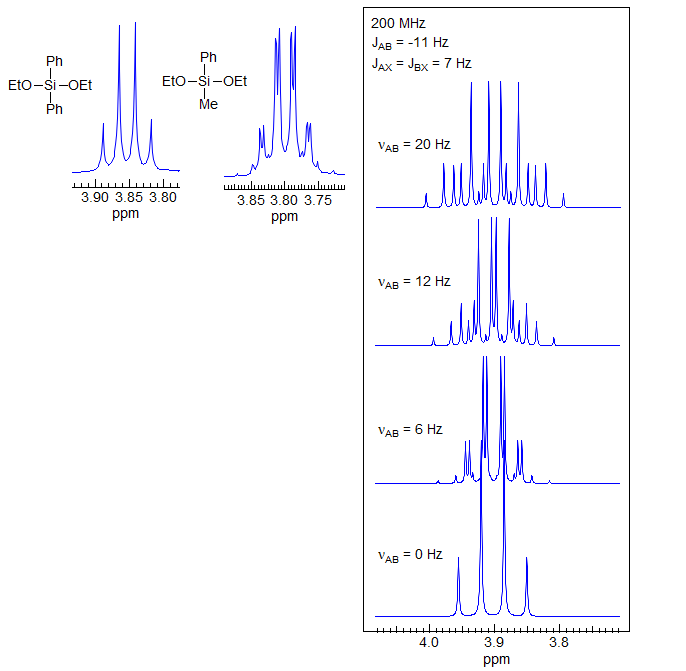
The simulated spectra shown mimic ABX3 patterns (AB part) of OCH2CH3 groups in chiral molecules. In these spectra all 16 of the lines are resolved, and recognition of the pattern is relatively easy. In real molecules it is common for several of the lines to be superimposed (especially since JAB is often nearly twice as large as JAX), making recognition of this pattern more difficult. In situations where the diastereotopic shift is small, the pattern can be mistaken for a quartets of doublets (see the νAB = 6 Hz spectrum).
It is not necessary for a molecule to have a center of chirality to show diastereotopic CH2 groups. Molecules with two ethyl groups attached to a prochiral center can also have ABX3 patterns, as illustrated in the spectra of the diethoxysilanes below. The left structure has enantiotopic CH2 protons, the right has diastereotopic ones (see Section 5-HMR-8 for the substitution test).
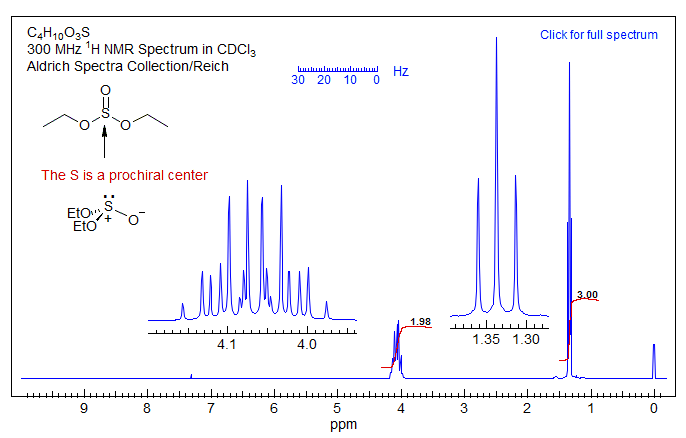
The spectrum of diethyl sulfite below is of historical interest - this type of diastereotopicity was first recognized for this molecule (Finegold, H. Proc. Chem. Soc., 1960, 283), with the correct explanation and analysis described in a classic paper (Kaplan, F.; Roberts, J. D. J. Am. Chem. Soc. 1961, 83, 4666), which also reported the first recognition that 2J and 3J at sp3 carbons have different signs. Diethyl acetals of aldehydes or diethyl ketals of unsymmetrical ketones also form ABX3 patterns.
Sample ABX3 Spectra
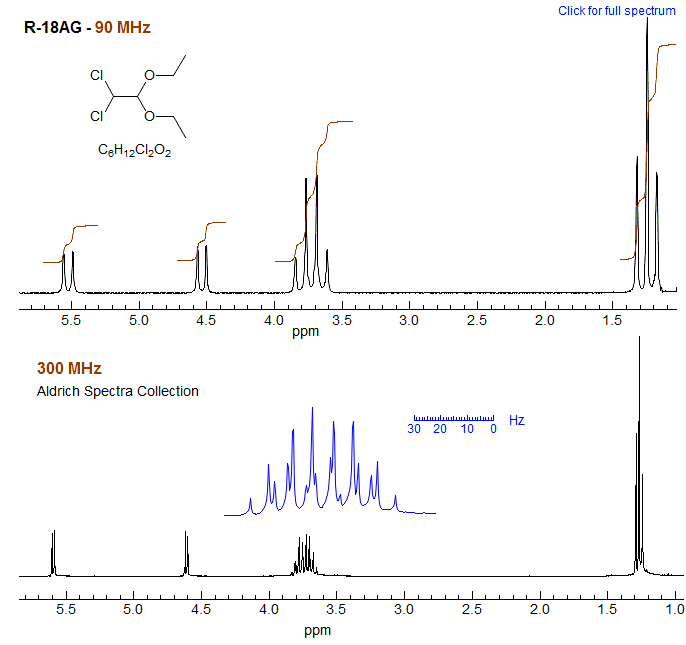
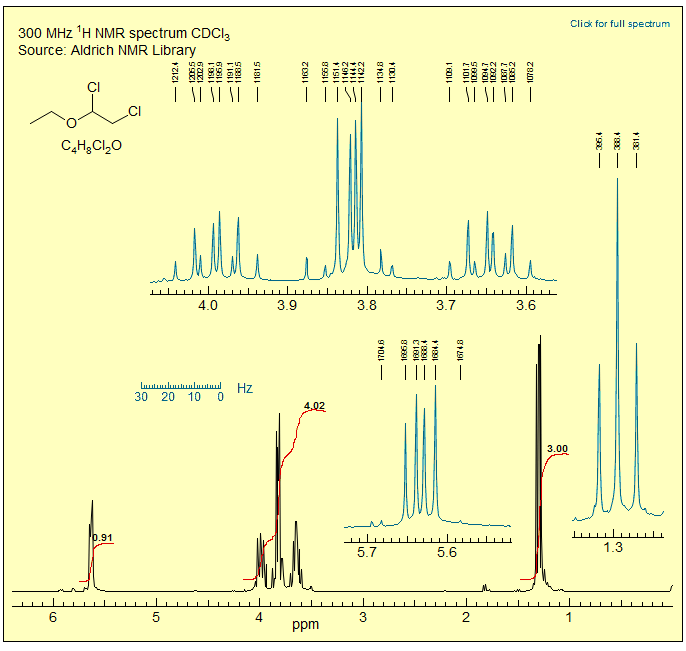
The spectra of dichloroacetaldehyde diethyl acetal illustrate that a molecule which contains diastereotopic protons can give decidedly simpler NMR spectra at low field than at high field. The CH2 group appears as a simple quartet at 90 MHz: the outer lines of the ABX3 pattern are too small to see, and the splitting of the inner lines is too small to resolve. At 300 MHz, on the other hand, the CH2 group is much more complex since HA and HB of the ethoxy are now far enough apart to give a well-developed ABX3 pattern.
Exercise: Analyze the spectrum below, determine δ and J values
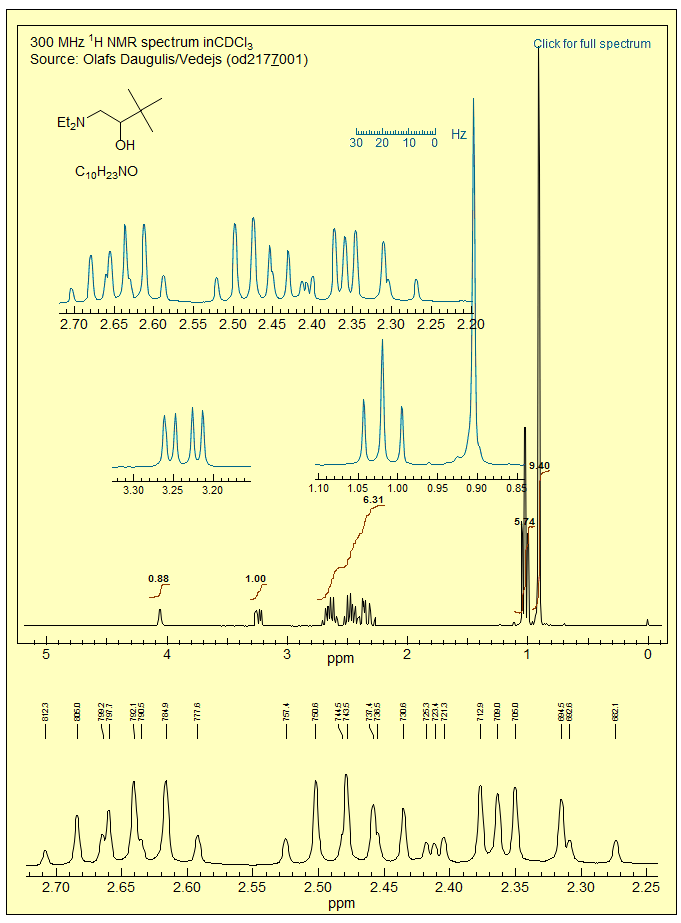
More ABX3 patterns:
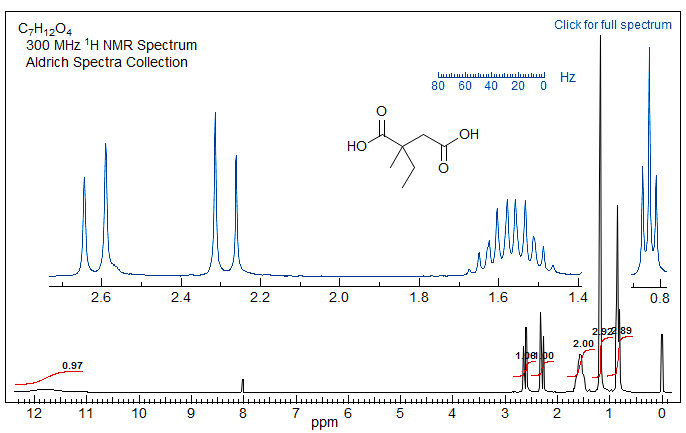
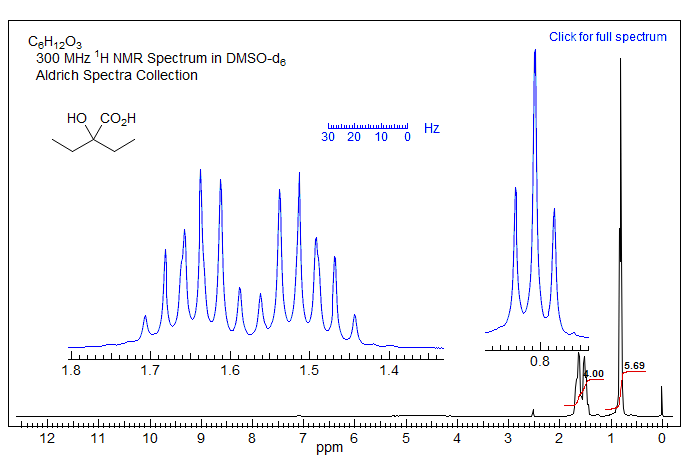
Exercise: Here is a test of the previous sections: analyze the spectrum below, assign all protons, identify all coupling constants.
The spectra below provide some details of a typical fully developed ABX3 system. If the CH3 group is decoupled, then we are left with a simple AB quartet, exactly analogous to AMX3 solution described above.
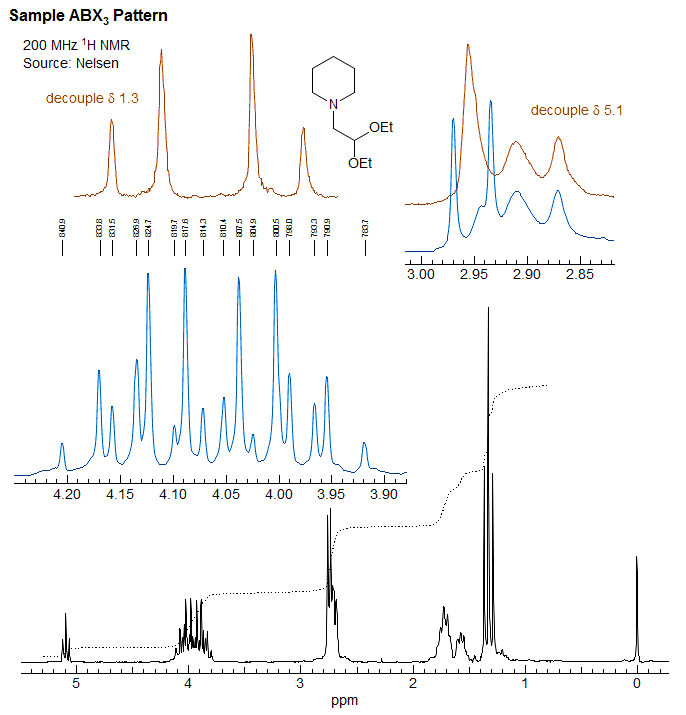
ABMX3 Patterns
Spin system where the CH2 protons of a diastereotopic ethyl group are coupled to a proton (ABMX3) or other spin =1/2 nucleus can also be readily understood and analyzed by a simple extension of the ABX3 analysis. The CH2 of the ethyl group is an AB quartet, each line of which is first split into quartets from coupling to the CH3 protons. The quartets are then split again into doublets by coupling to M, giving a ddq for each of the CH2 protons. Some structure fragments which give such patterns are shown below. For 2 the M nucleus is the spin 1/2 phosphorus. In each case R1 and R2 must be different to make the CH2 group diastereotopic. Examples: 1, 2, 3, 4.
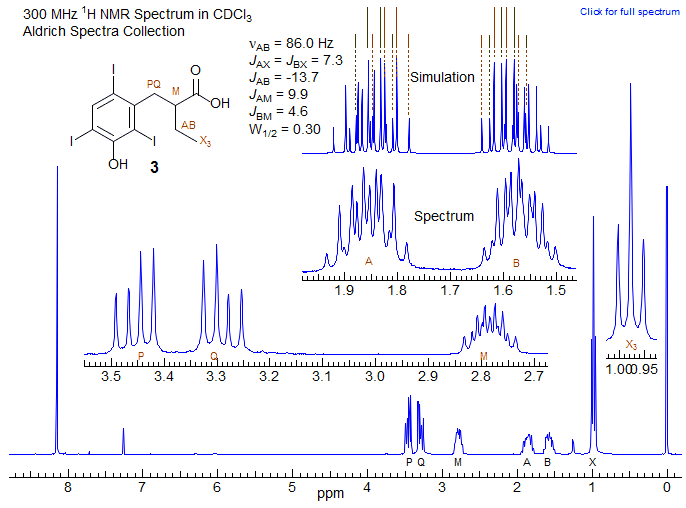
The spectrum of 3 below is of this type. One of the two dq of each proton is shown schematically above the simulation, which is plotted with a narrow line width so all of the lines can be resolved. Note that in this case the coupling of A and B to the X3 protons are identical, but A and B are coupled differently to the M proton. The M proton in addition also coupled to two others labeled P and Q.
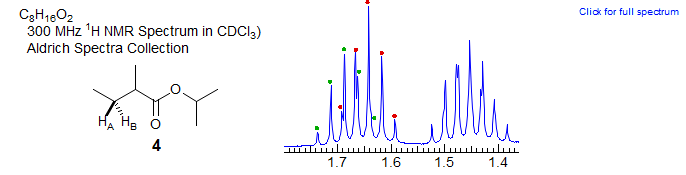
It is fairly common for ABMX3 patterns of the CH-CHAHB-CH3 type (1) to show nearly equal JAX, JBX, JAM and JBM. In this case the CHAHB group appears as an AB quartet of pentets. The partial NMR spectrum below shows the β CH2 signal of isopropyl 2-methylbutyrate (4). The downfield signal is a clean doublet of pentets, the upfield one closer to a ddq.
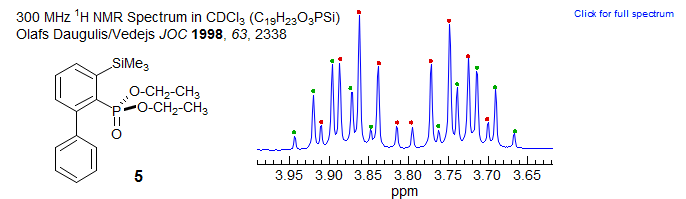
The phosphonate 5 has the diastereotopic OCH2 protons coupled equally to the CH3 group and the phosphorus, so here also a dp is seen for each proton (AB quartet of pentets). Related Examples: 1, 2.
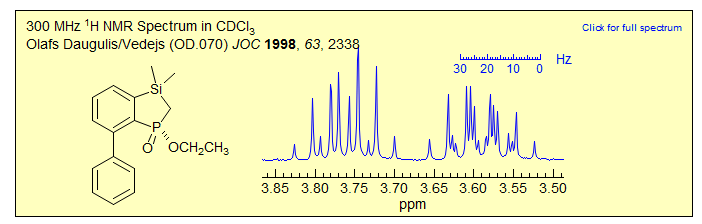
Exercise: Assign the protons and completely solve the pattern below,
Next Section: A2X2 and A2B2 Patterns · Previous Section: ABX Patterns · Home