5-HMR-15 The AA'BB' Pattern
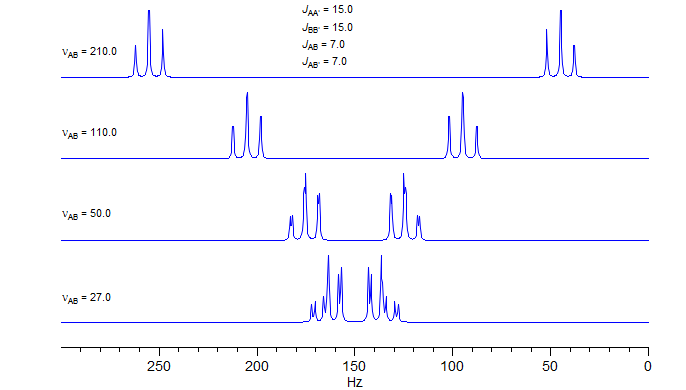
As A and X of the AA'XX' spectrum move closer together, the lines of the 1:1 doublet (N) each split into two lines, for a total of twelve in each half-spectrum. The AA' and BB' parts are no longer centrosymmetric, but develop a mirror image relationship, so that the entire pattern is centrosymmetric. As is found for all AX to AB transformations, "leaning" occurs, the inner lines increase and the outer lines decrease in intensity, culminating in just a single line when νAB = 0. AA'BB' patterns can be solved manually, but the process is difficult, and is better done with computer simulations.
Typical molecular fragments which give AA'BB' patterns are:
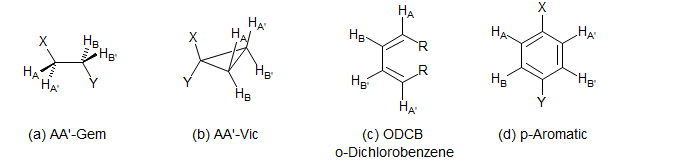
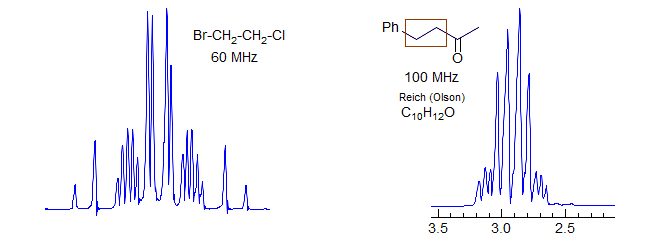
(a) AA' Geminal (X-CH2CH2-Y).
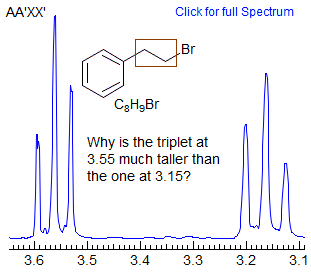
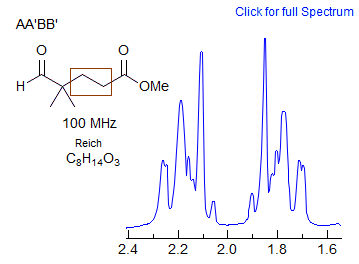
This is the most common type of AA'BB' pattern. The appearance can range from essentially perfect triplets, to rather complicated patterns which cannot be easily analyzed. The two spectral parameters which control appearance of the spectrum are chemical shift difference νAB, and the difference between the two vicinal coupling constants JAB and JAB'. If νAB is small (AA'BB'), then the pattern will always be complicated, no matter what the coupling constants are. If JAB ≈ JAB' then the pattern will mimic A2X2/A2B2 and triplets will be seen if the chemical shift is large enough. Examples: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
The key feature that determines the complexity of AA'BB' patterns of CH2-CH2 groups is the relative size of JAB and JAB', which is determined largely by the conformational properties of the X-CH2CH2-Y fragment. For acyclic systems, if the X and/or Y groups are small, then the populations of the anti and gauche conformations will be close to statistical (1:2). As can be seen from the table and the simulated spectra below, the two averaged couplings become approximately equal when there is 67% of gauche isomer, and the spectrum will mimic an A2B2 pattern -- triplets if νAB is large enough (νAB >> JAB). If X and/or Y are large, then the anti isomer will be favored and the pattern will be more complex. In practice, adjacent CH2 groups often look like triplets, and thus the gauche conformation must usually be favored. For cyclic systems (e.g., N-cyanomorpholine) the ring constrains the -CH2CH2- fragment to mostly the gauche conformation, and clean triplets are not usually seen.
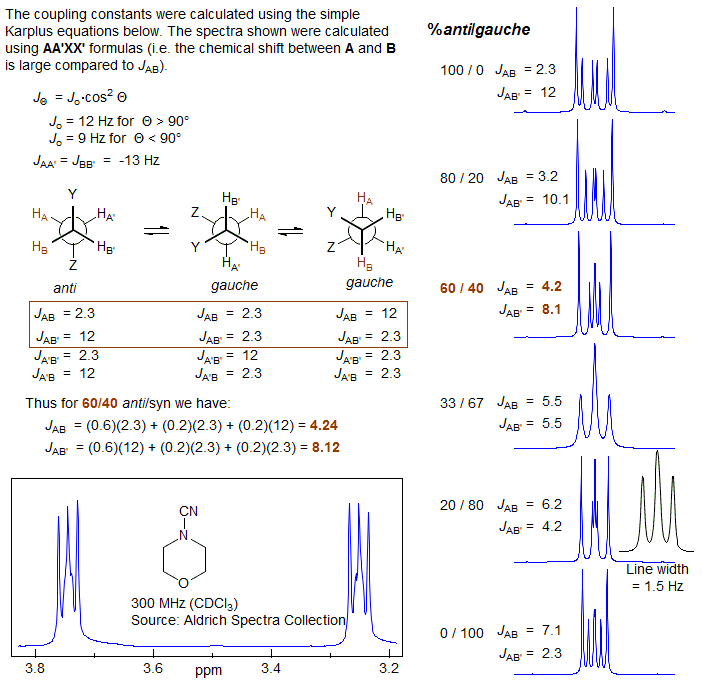
It is a common misconception that the equalization of coupling constants (and hence the appearance of triplets) is a consequence of free rotation around the CH2-CH2 bond. In fact, there is free rotation around almost all such bonds in acyclic molecules at accessible temperatures. The appearance of more complicated patterns is the result of a preference for the anti conformation over the gauche (or vice versa), and has nothing to do with the rate of rotation. See section 5-HMR-09 for some additional examples.
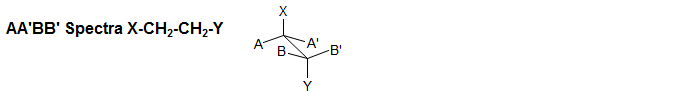
(b) AA' Vicinal.
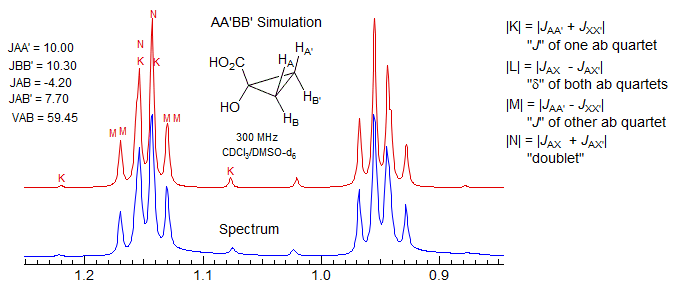
This type of AA'BB' pattern is much less common than type (a). It appears principally in 1,1-disubstituted cyclopropanes, 2,2-disubstituted 1,3-dioxolanes and other similar structures. The patterns are never triplets because JAB is invariably quite different from JAB'.
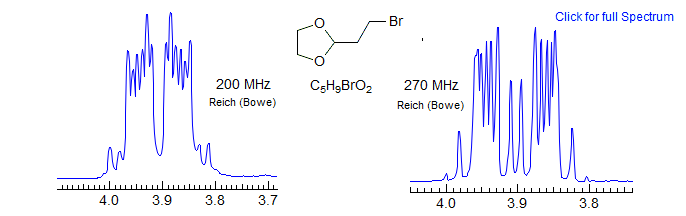
The N doublet peaks are close together in these patterns, often inside the K and M ab quartets, because JAX is negative, and JAX' is positive (N = JAX + JAX'). Since JAA' ≈ JXX' the K ab quartet has very small outer peaks, and the inner peaks will then be close to the N doublet peaks. Similarly the "ab" M quartet will have a near zero "coupling" (M = JAX + JAX') and so will be essentially two singlets, or two very closely spaced doublets, not resolved in the spectrum below. These features can be seen in the dioxolane spectrum, which has been simulated using the parameters shown (red trace). Peak labels are for the AA'XX' assignments. Since this is actually an AA'BB' pattern, the N lines each split into two closely spaced ones. Because the A/X shift difference is diastereotopic in nature, most of these patterns tend toward AA'BB' except at very high field strength. Examples: 1, 2 , 3, 4.
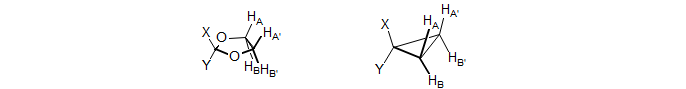
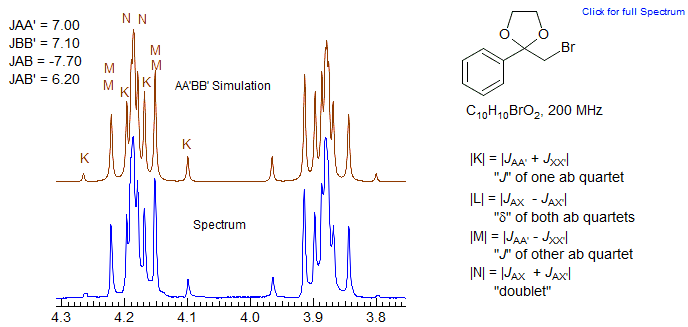
Here some AA'BB' spectra of dioxolanes.
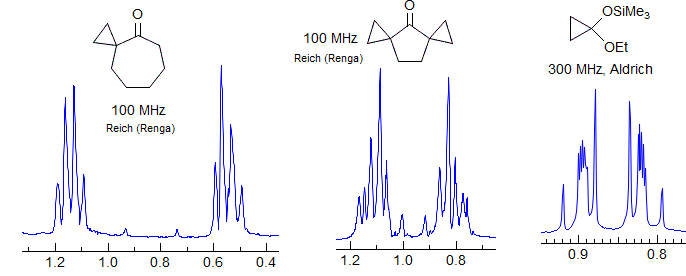
Unsymmetrical 1,1-disubstituted cyclopropanes often have AA'XX' patterns that have a quartet-like appearance. Because JAA' is close to JAX', the M quartet is essentially a doublet, and the L quartet is very strongly leaning (see the spectra and simulation below). This means that the central two line clusters have essentially 3/4 of the intensity (N+K), and the M lines 1/4, just as for a regular quartet
The half-spectrum of 1-phenyl-1-cyanocyclopropane illustrates this effect. Here JAA' and JXX' are a little more different than in the above example so the M quartet is visible as four lines. This 2-proton multiplet could be mistaken for a doublet of quartets by the unwary spectroscopist.

(c) o-Dichlorobenzene (ODCB) Type.
This kind of AA'BB' pattern never approaches A2X2 because JAB (ortho coupling) is always much larger than JAB' (meta coupling). It is seen in symmetrically 1,4-disubstituted dienes and ortho disubstituted benzenes. Note that for all AA'XX' / AA'BB' systems the A and X / B patterns are identical (although they have a mirror-image relationship). This is in spite of the fact that the coupling relationships of A and X / B are often quite different. The spectra often have the appearance of a dd.
For most AA'XX' patterns of this type JXX' is a 3-bond coupling, and JAA' a 5-bond coupling, Thus the K and M ab quartets will be nearly superimposed (the Jab values differ by 2JAA'), often leaving only 6 resolvable lines (Simulation A, naphthalene, o-dichlorobenzene). Even with the small JAA' values that are usually seen, the central lines of the K and M quartets will likely be superimposed, whereas the small outer lines may be distinct -- they will be separated by just under twice the value of JAA', the inner lines by just a fraction of JXX' (Simulation B, spectrum of biphenylene and the 1,4-diacetoxybutadienes). Examples: , 1, 2, 3
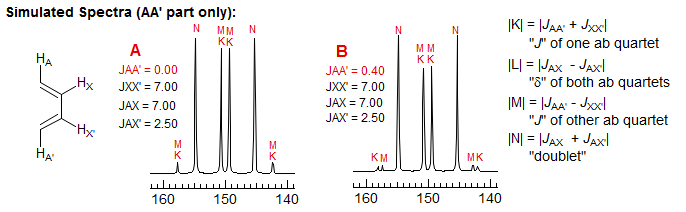
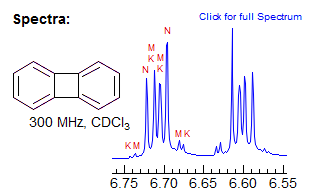
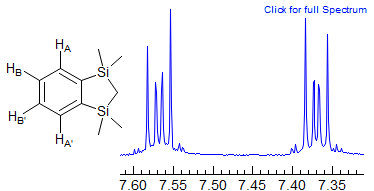

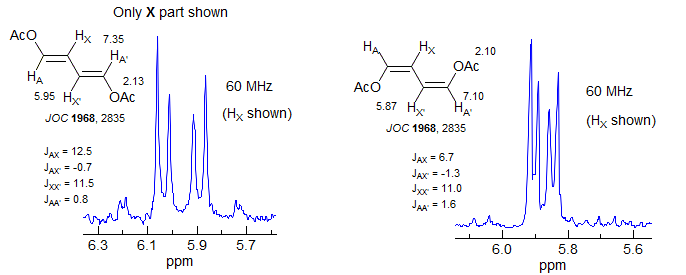
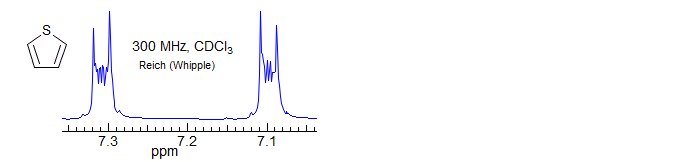
As with all such NMR spectra, the patterns get more complex when the chemical shift between the protons becomes smaller (AA'BB'), and the parameters can no longer be extracted by a simple analysis. Some examples (both A and B shown):

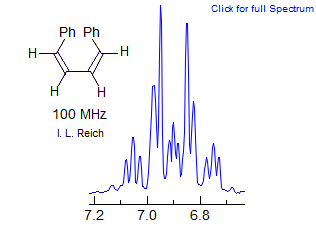
(d) p-Disubstituted Benzene Type.
These usually resemble two AX doublets or an AB quartet, with some small extra lines.
For AA'XX' patterns of this type, the two 3J meta couplings (JAA' = JXX') are likely to be very close in size so that the M ab quartet collapses to two lines since M = 0 or very small (Simulation B). In addition, the para-coupling (JAX') is nearly zero, which makes L ≈ N. Thus the two lines of the M quartet will appear at or close to the chemical shift of the N doublet, leaving only a doublet and one ab quartet, whose doublets will be on both sides of the N+M lines (simulation A). This is why p-substituted benzenes are often incorrectly described as doublets, since the extra lines corresponding to the K quartet amount to only 1/4 of the total intensity, and appear fairly close to the dominant M+N doublet.

When the chemical shift between A and B is small (as in p-bromochlorobenzene below), the usual leaning effects are seen, additional lines appear, and the extra lines become more pronounced. As 300 MHz a more "normal" AA'BB' pattern is seen

(e) Miscellaneous AA'BB' Patterns.
In the 1,3-substituted cyclobutane below the 4JHH (JAA', JBB' and JAB') are large enough that the expected first-order AB quartet is not seen, but a much more complicated pattern. In this case, as in the p-disubstituted benzene case above the "AB" character dominates the appearance of the multiplet, largely because the coupling interactions between either of the AB protons and the A'B' protons are much weaker than the A to B and the A' to B' couplings (i.e. the AB pair is nearly isolated from the A'B' pair). Another example: 1.
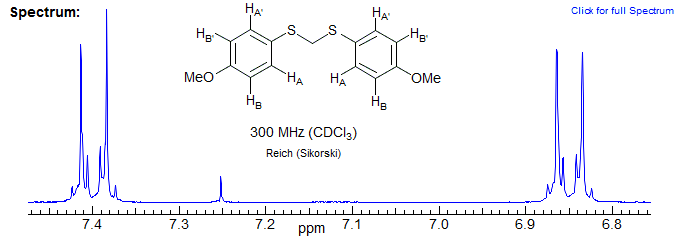
Symmetric trans-1,2-disubstituted cyclopropanes can form AA'BB' patterns. Some examples are shown below. Note the identity of the A and B multiplets, even though they are in very different environments.

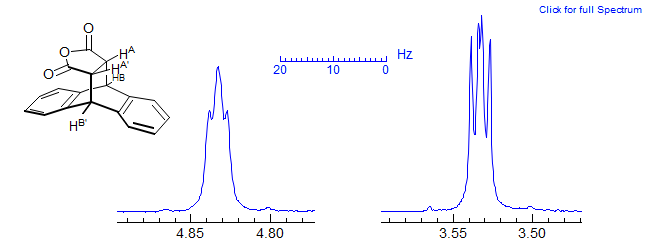
Yet another type of AA'XX' pattern is shown in the Diels-Alder adduct below. Even though the two sets of protons are in very different environments, the two patterns are identical except for some broadening of the benzylic protons at δ 4.83 due to long range coupling to the aromatic protons. The aromatic protons of this compounds are two superimposed AA'BB' patterns of the ODCB type.
Larger symmetric spin systems also show highly complex patterns. Below is an example of an AA'A''A'''BB'B''B''' system in a pyrrolidine ring.
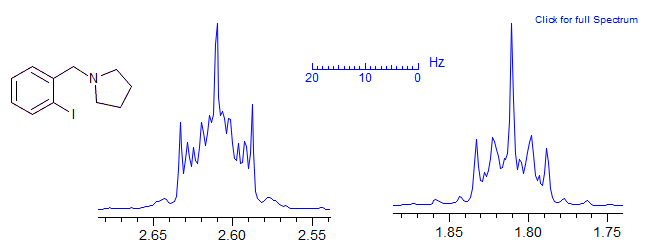
Origin of Complexity in Patterns of the AA'XX'... Type
There are two situations where spin systems containing AA'XX' type do not show unusual complexity. One is where JAX = JAX', in which case the pattern becomes first order A2X2.
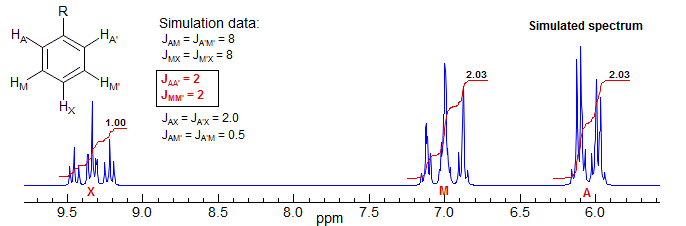
The second is systems in which there is no coupling between both of the chemical shift equivalent protons, i.e., JAA' = JXX' = 0. In such cases the degeneracy between spin states is no longer present, and first order systems result. Consider two examples. A monosubstituted benzene is an AA'BB'C or AA'MM'X system. A simulated spectrum is shown below
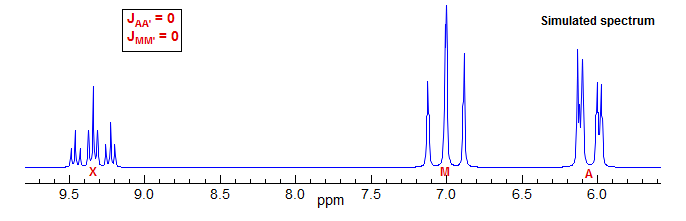
If we recalculate the spectrum after setting the meta couplings JAA' = 0 and JMM' = 0 then it becomes essentially first order (it would be completely first order if the chemical shifts between A, M and X were made larger).
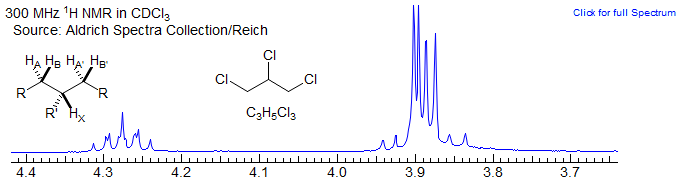
For this reason, some spin systems which are formally of the AA'XX' type, but in which there is no significant spin-spin coupling between the equivalent protons show first order spectra. For example, the fairly common spin system below of the AA'BB'X type shows no unusual complexity (beyond that of normal ABX patterns) because there is no significant coupling between the A and A' protons, nor between the B and B' protons. Such systems are sometimes defined as (AB)2X to indicate that magnetic inequivalence is not a factor. The spectrum of 1,2,3-trichloropropane is an example. Examples: 1, 2, 3, 4, 5.
Contrast this with the NMR spectrum of dibromosulfolane below.
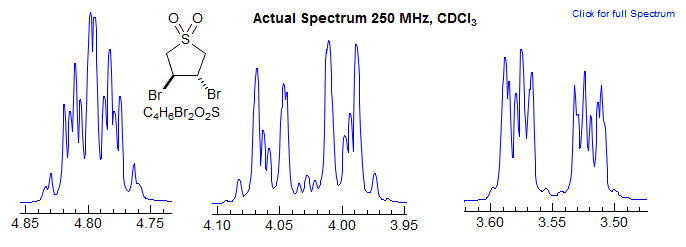
Why is this spectrum so complex? In this AA'MM'XX' system, although there is no significant coupling between A and A' or M and M', there is coupling between X and X', making the whole system a highly second order one (remember: anything coupled to a strongly coupled pair like the XX' protons becomes second order).
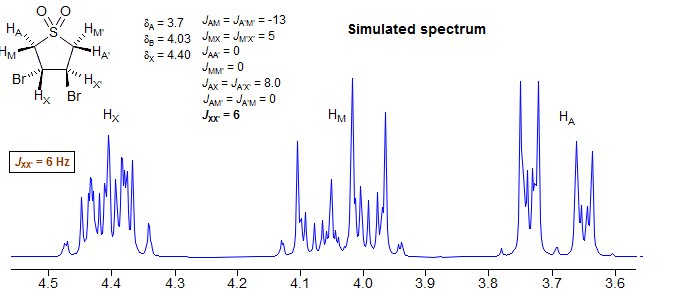
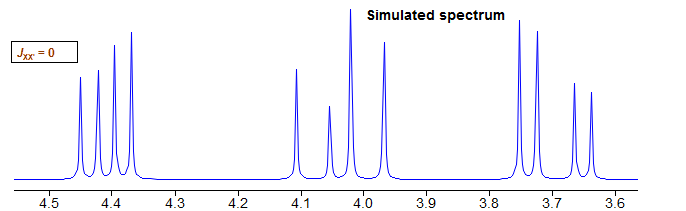
If we remove the XX' coupling, the system becomes essentially first order (two isolated AMX patterns). We could better describe it as an (AMX)2 rather than an AA'MM'XX' spin system.
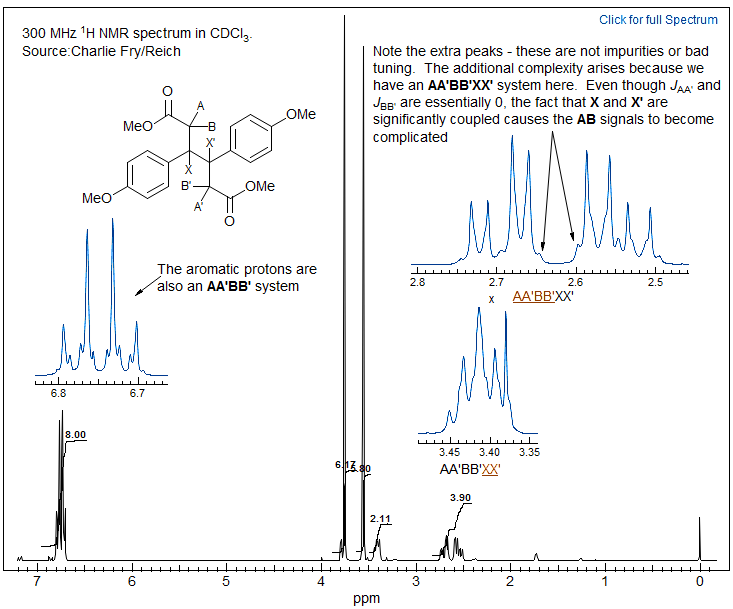
Another example of the same spin system is shown below.
Next Section: Virtual Coupling · Previous Section: ABX3 Patterns · Home