6-CMR-4 13C Chemical Shift Effects on sp2 and sp Carbons
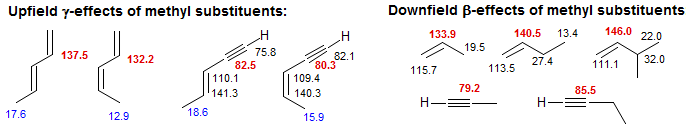
The carbons of double and triple bonds also show the α, β and γ effects which have been well established for saturated carbons. In the examples below, both the sp2 and sp carbons show upfield γ-effects, and downfield β-effects, similar in magnitude to those seen for sp3 carbons.
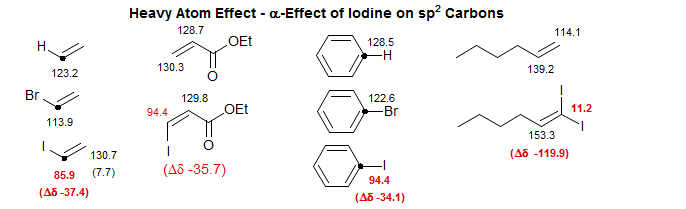
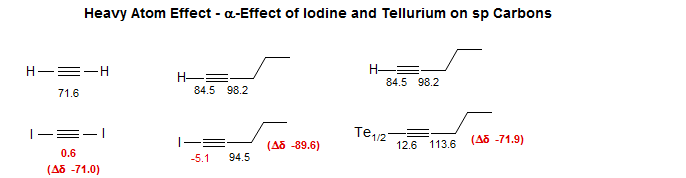
There are also heavy atom α-effects, seen mostly for carbons bonded to iodine Iodoacrylate, Iodoalkene, Iodonitrobenzene, Iodopentyne, Telluropentyne.
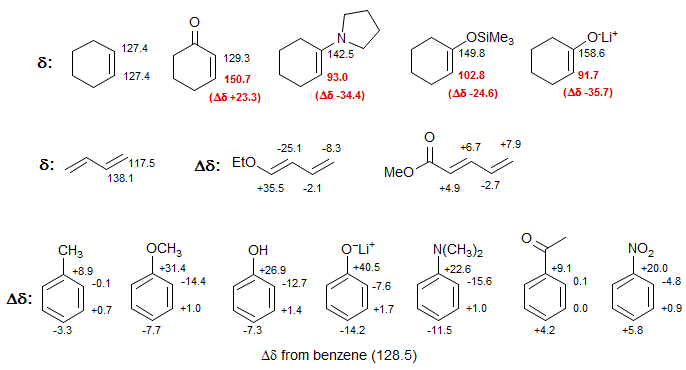
1. Conjugation with π-Acceptors and π-Donors
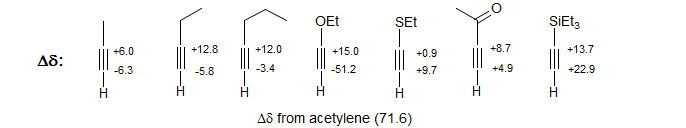
Vinyl and alkynyl carbons show large charge density effects resulting from partial positive and negative charges in the π-system. Chemical shifts in π-polarized double and triple bonds follow charge densities in a reasonable way as qualitatively predicted by drawing resonance structures. Thus the β-carbon of α,β-unsaturated carbonyl compounds is downfield, whereas those of enol ethers and enamines are strongly upfield.
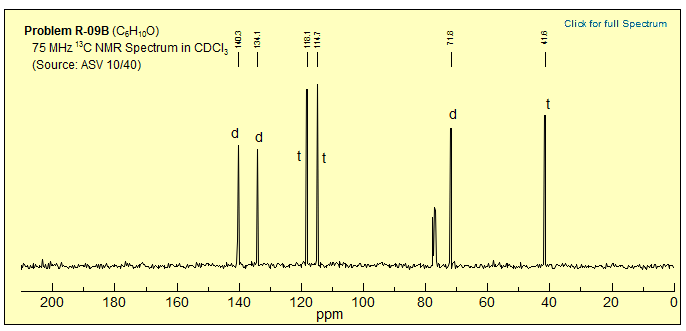
Exercise: Determine the structure of the C6H10O isomer from the 13C NMR spectrum below.
Alkynes with first-row element substituents are also polarized in the same sense. However, with second and third row elements more complicated chemical shifts effects come into play.
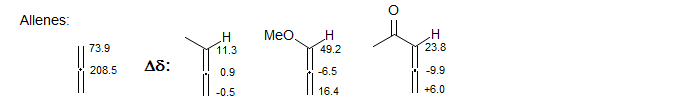
2. Strongly Charged Systems
Carbanions, carbonium ions. Must be very careful here. If charge is localized (σ-charge, sp3 systems), effects are quite variable, and frequently the opposite of those in π-systems (e.g., C-1 of PhLi is at δ 171.9, CH3Li δ -13.2).
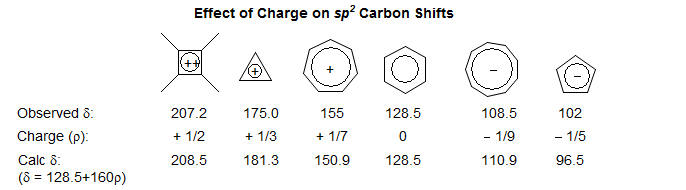
On the other hand, if charge is part of a π system, chemical shifts follow charge density rather well (160 ppm/electron). The various monocyclic aromatic anions and cations show a remarkably consistent set of chemical shifts, which can be accurately predicted from the excess charge density at each carbon using the formula shown (Prog. Phys. Org. Chem 1976, 12, 229).
3. Carbonyl Groups
Carbonyl groups appear in two regions: ketones and aldehydes from 190-220 ppm, esters, acids amides and related carbonyl functions between 150 and 175 ppm. The ketone region is quite distinctive, the only reasonably common function that appears around 200 ppm is the central carbon of allenes. There are more interferences in the acyl-X region, with occasional double bond and aromatic sp2 carbons, as well as C=N carbons appearing in the same range. Unfortunately, the various carboxylic acid derivatives do not have distinct chemical shifts ranges, so that acids, esters, acid chlorides, amides, anhydrides are not readily distinguished in the 13C NMR spectrum (this can often be done by examining the carbonyl stretch in the IR spectrum). Even carbonates, ureas, and carbamates are not well separated from the carboxylic acid derivatives.
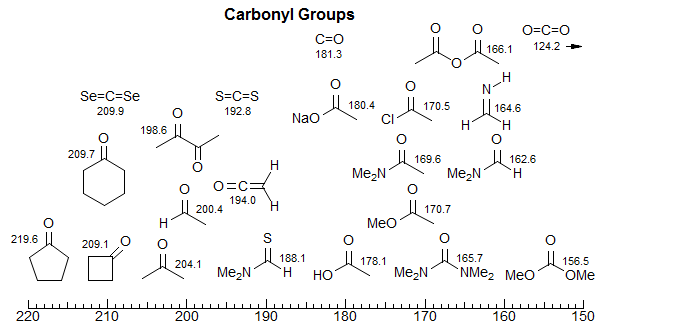
There are several chemical shift effects of carbonyl groups which are large and consistent enough to be useful for structure assignment.
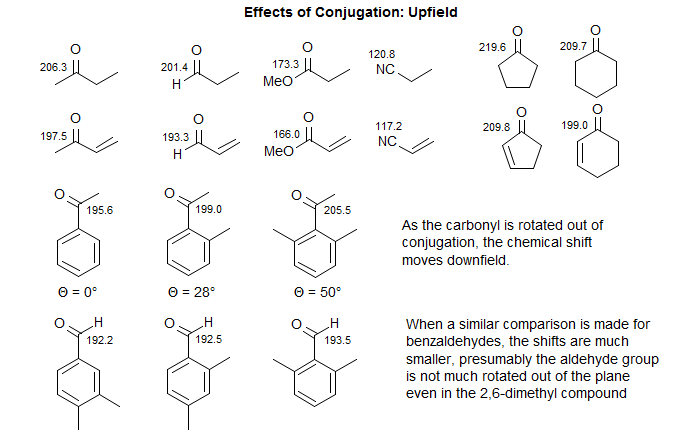
Conjugation Effects. Conjugation to a double bond or aromatic ring causes upfield shifts (smaller δ value) of 6-10 ppm for all types of carbonyl compounds. The effect is smaller for nitrile carbons, but in the same direction.
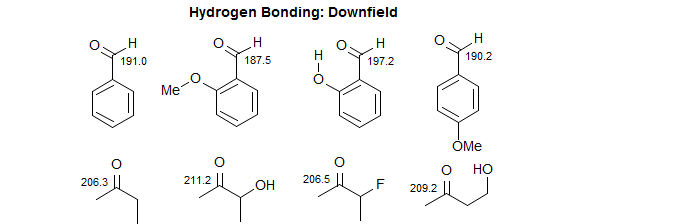
Hydrogen Bonding Effects. Intramolecular hydrogen bonding causes substantial downfield (larger δ value) shifts. Most carbon signals are quite insensitive to solvent effects. Carbonyl groups are an exception – they move downfield in protic solvents, an effect also attributed to hydrogen bonding.
Olefinic Carbon Shifts
A set of parameters for estimation of the sp2 13C chemical shifts of hydrocarbon alkenes has been developed (Roberts et al., J. Org. Chem. 1971, 36, 2757; F. W. Wehrli and T. Wirthlin, "Interpretation of C-13 NMR Spectra", Wiley, 1974, p. 41). Note that α' is beta to the carbon being calculated, β' is gamma.
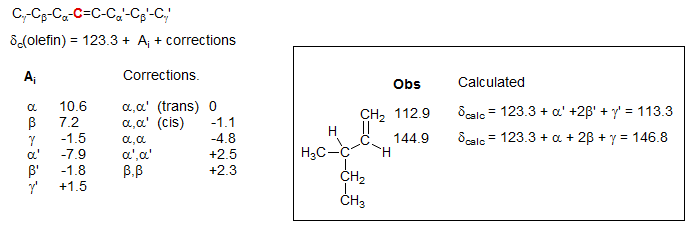
There are quite characteristic differences between the two olefinic carbons as a function of substitution:
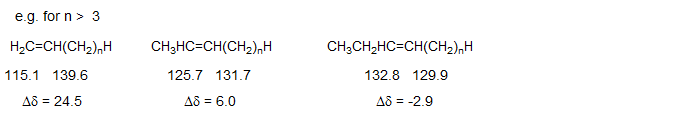
Nitrile Carbon Shifts
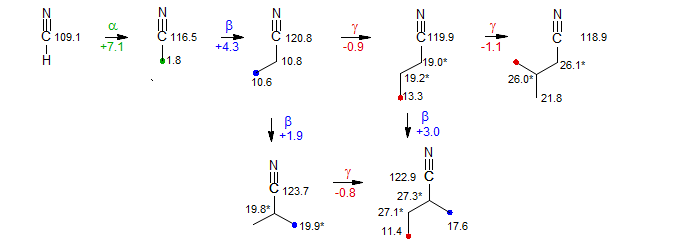
Nitrile carbons are almost always well separated from the remaining carbons, and thus easily identifiable. Chemical shift effects on the CN carbon follow the pattern for sp3 and sp2 carbons, and can be structurally diagnostic. β-Effects are downfield but relatively small (+2 to +4 ppm). The upfield γ-effect is also small (about -1 ppm), but is consistently seen, as illustrated in the graphic below.
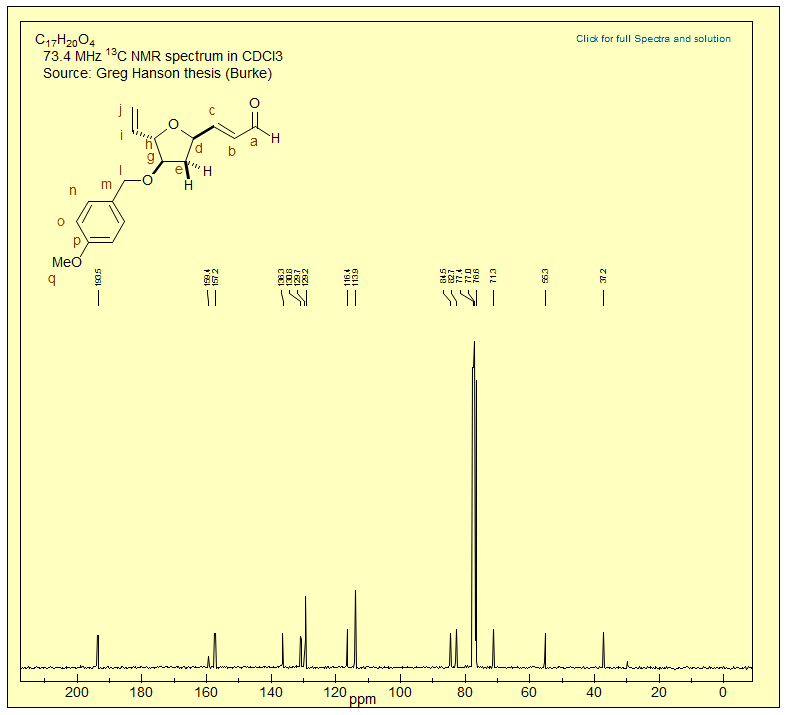
Exercise: Assign all of the carbons in the 13C NMR spectrum below.
Next Section: 1JC-H · Previous Section: Shifts ·Home