6-CMR-6 Two and Three Bond Carbon-Proton Couplings
Couplings between carbon and protons across two and three bonds are generally small, and can be both positive and negative. For additional data see J(C-H) data. They show many of the trends found for H-H couplings (although they are typically smaller), including the effects of π and σ donors and acceptors for 2J, and the Karplus relationship for 3J. For examples using ~3^IJ^i|C|H to determine stereochemistry see: 1, 2, 3.
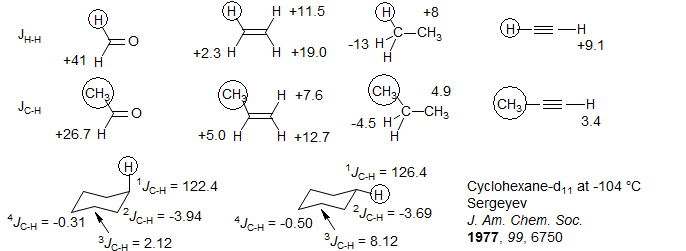
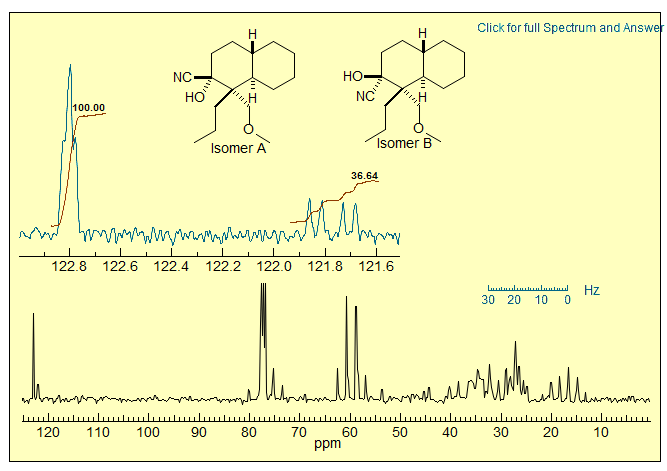
Problem R-13I (C16H27NO2). Determine the stereochemistry of a ≈1:3 mixture of two isomers of a cyanohydrin from the fully coupled 75.6 MHz 13C NMR spectrum (CDCl3) shown below. Source: R, Corcoran, U. Wyoming.
A very useful effect is the reliable difference in cis and trans C-H couplings across double bonds (or partial double bonds, such as those in esters and amides), which allows assignment of stereochemistry in situations where H-H couplings are not available. It is difficult to measure these couplings from observation of 13C satellites in proton spectra, so they are normally measured from coupled 13C NMR spectra, or in 13C enriched compounds. It is important when applying this technique to recognize that any given carbon may be coupled to a number of other protons. Thus some means of recognizing the coupling to the proton of interest must be available, either from the size of J or multiplicity of the coupling pattern, or from NMR techniques such as single frequency proton decoupling or 2D heteronuclear correlation experiments. Examples: 1, 2, 3.

To determine stereochemistry this way it is desirable to have both isomers, because the cis and trans coupling ranges do overlap.

Three-bond proton-carbon couplings, when available, follow a Karplus relationship much like H-H couplings, and can be used for conformational and configurational assignment in saturated systems.(Review: "Determination of Relative Configuration in Organic Compounds by NMR Spectroscopy and Computational Methods" Bifulco, G.; Dambruoso , P.; Gomez-Paloma, L.; Riccio, R. Chem. Rev., 2007, 107, 3744 DOI: 10.1021/cr030733c).
The 2JCH and 3JCH within benzene rings can provide unambiguous chemical shift assignments for closely spaced aromatic carbons. For example, the ortho and meta carbons of many monosubstituted benzenes have the same intensity, are often close in chemical shift, and can be difficult to assign. The only substantial JCH coupling within a benzene ring is the meta 3-bond coupling (typically around 7 Hz), the others are small. An ortho carbon will have two protons meta to it, thus a dt, whereas a meta carbon, will only have one proton meta to it, hence only a dd. It should be noted that in detail these dd and dt patterns are actually more complex, since they correspond to the X nucleus of AA'BB'CX patterns, which are never strictly first order

As can be seen in the fully coupled 13C NMR spectrum of butyrophenone below, one of the carbons between 128 and 129 ppm is a doublet of doublets, hence the assignment to the meta carbon, the other a doublet of triplets, the ortho carbon. One might have made the opposite assignment based on resonance effect arguments. The para carbon is also a dt, although the assignment there is easy because of the size of the peak. The ipso carbon is a narrow triplet. Other examples: 1

Of course, this strategy can also be used to help with the assignment of individual carbons in more highly substituted aromatic rings.
Next Section: 4JC-H · Previous Section: 1JC-H ·Home