7-MULTI-3 NMR Spectroscopy of Spin ½ Nuclei other than 1H and 13C
7-MULTI-3.1 100% Abundance Nuclei
For those nuclei with high natural abundance, such as 31P and 19F, high resolution spectra are easy to obtain, and are routinely used by chemists and biochemists working with phosphorus and fluorine compounds. In fact, one can argue that the expense of doing research with F and P compounds is reduced, and the scope, volume and reliability enhanced because the NMR properties are so favorable. Fig. 7.3.1 shows the proton decoupled 31P NMR spectrum of a platinum complex.Other 31P NMR examples: 1, 2, 3, 4, 5, 6. 19F NMR examples: 1, 2, 3.
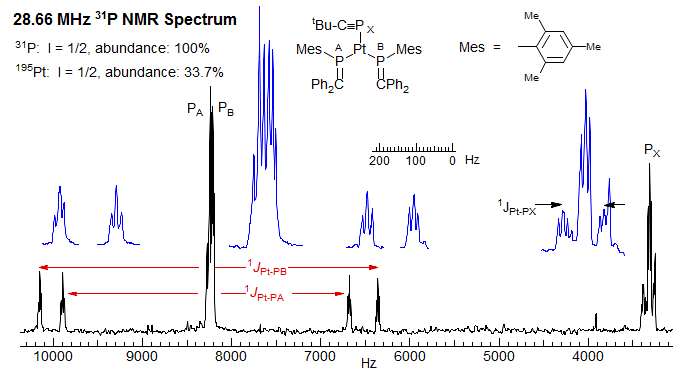
Figure 7.3.1. 28.7 MHz 31P 1H NMR spectrum. Note that PA and PB are very close together in the central peak, but well separated in the Pt satellite spectra, because the differences in chemical shifts of PA and PB are smaller than the differences in PA-Pt and PB-Pt couplings (J. F. Nixon, Chem. Commun. 1983, 930).
Exercise: The 31P NMR spectrum of one of the phosphorus atoms of (CF3)2P-PH2 is shown below. Which P is it? Extract coupling constants (J. Fluorine Chem. 1972/73, 2, 276).
Especially 19F NMR spectra are easily obtained since the proton channel on most spectrometers can be tuned to 19F, and thus little additional hardware is required. Fluorine chemical shifts are very sensitive to changes in structure and environment and show no interference from solvents. Thus fluorines are often incorporated into substrates to be used as "reporter" nuclei to follow molecular events in situations where 1H and/or 13C spectra may be hopelessly crowded or insufficiently sensitive. An example is shown in Fig. 7.3.2 where the 19F NMR spectra of a para-fluorine label were used to probe the solution structure of a lithium phenoxide.

Figure 7.3.2. 139.96 MHz 7Li (I = 3/2, Q = 0.02) and 338.8 MHz 19F NMR spectra at -120 °C of an HMPA titration of a tetrameric lithium phenoxide with para-fluorine label as a "reporter" nucleus. The sequence of species and their NMR signals as HMPA coordinates to each of the lithiums can be clearly identified. The 19F signals go in sequence from 1:0 to 1:3 to 1:1, 3:1 and to 1:0 peaks for the consecutive solvates. The corresponding 7Li signals show doublets due to 7Li-31P coupling for the Li-O=P(NMe2)3 signals and singlets for the others. These spectra show unambiguously that the phenoxide is a tetramer which sequentially solvates all four lithiums. The spectra are not proton decoupled, at higher resolution each fluorine peak is a triplet of triplets (Kolonko, K. J.; Biddle, M. M.; Guzei, I. A.; Reich, H. J. J. Am. Chem. Soc. 2009, 131, 11525–11534).
7-MULTI-3.2 Low Abundance Spin ½ Nuclei
Except for P, F, Rh and Y, the natural abundance of the commonly observed spin ½ nuclei such as 13C, 15N, 29Si, 77Se and 119Sn is less than 10%. Thus sensitivity is a problem for many nuclei. Since the inherent sensitivity is a strong function of the gyromagnetic ratio, the sensitivity of all nuclei except 3H is less than that of 1H. For very low frequency nuclei (15N and below) there are also instrumental problems.
Chemical shifts (δ) and chemical shift effects (Δδ) of all types are much larger for heavier elements than for 1H and 13C. Shift ranges of 2000 to 20,000 ppm are not uncommon. The temperature dependence of chemical shifts can be quite large and a significant source of peak broadening when sample temperature is not homogeneous. For example, 77Se shifts can change as much as 1 ppm/°C. Thus a 1° gradient across the sample will give a 95 Hz linewidth (on a 500 MHz instrument). Such problems have also been encountered with 125Te, 119Sn and 199Hg.
7-MULTI-3.3 Isotope Shifts
Isotope shifts are large and often easily detectable. The largest mass change possible is from H to D, and this substitution typically gives easily observable isotope shifts, even over several bonds. Some of the largest ones observed (other than special situations involving Saunders equilibrium isotope shifts) are shown below in the spectra of SnH3- and deuterated analogs (Fig. 7.3.3).

Figure 7.3.3. 119Sn NMR spectra of SnH3- and deuterated analogs in liquid ammonia at 20 °C (Wasylishen, J. Chem. Soc., Chem. Commun. 1987, 1414, spectrometer frequency not given).
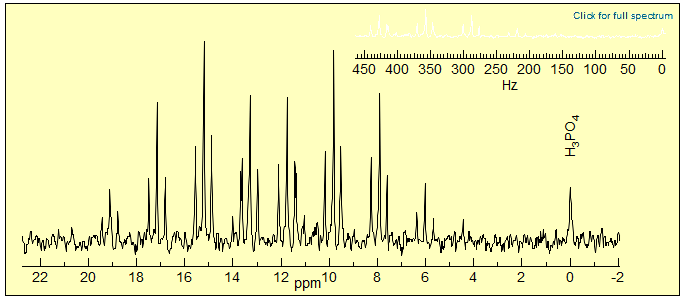
The isotope shift method provides a technique for the detection of NMR-transparent nuclei such as 18O. For example, the 31P NMR spectrum of a multiple-18O labeled sample of ATP (adenosine triphosphate) in Fig. 7.3.4 allows accurate measurement of numerous differentially isotopically labeled compounds.
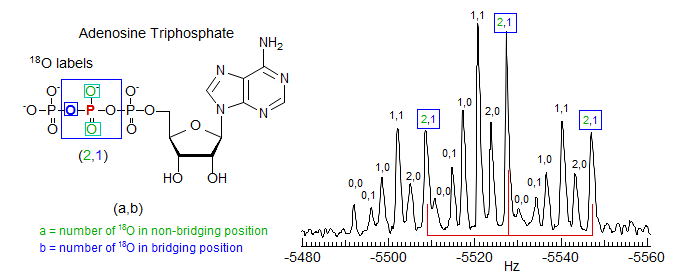
Figure 7.3.4. 235 MHz 31P NMR spectrum of the β-P of 63% 18O ATP-β,γ-18O (29000 scans) . Each isotopomer gives a triplet, offset by 16O/18O isotope shifts. The shifts are larger for the stronger and shorter P=O bond than for P-O-P. (Cohn, M.; Hu, A. J. Am. Chem. Soc. 1980, 102, 913).
Selenium-77 NMR chemical shifts are very sensitive to environment. Selenocarbonyls are especially so, covering a range of ca 2600 ppm. This property is potentially useful for detecting stereochemical and isotopic labeling of molecules. One system that has been developed (L. A. Silks, III, R. B. Dunlap, J. D. Odom J. Am. Chem. Soc. 1990, 112 ,4979-4982) is a selenocarbamate derived from nor-ephedrine. The derivative with α-deuterated phenylacetic acids shows a remarkable separation between the undeuterated, mono- and dideuterated isotopomers, and even the two epimers of the mono-deuterated compound! This for a D located 5 bonds from the Se.
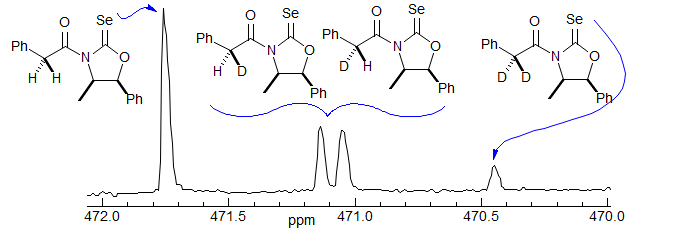
Figure 7.3.5. Selenium-77 NMR spectra of a D-labelled selenocarbamate used to establish optical and isotopic purity.
Sometimes the entire chemical shift range cannot be covered in a single experiment (depends on spectrometer - modern spectrometers have sweep width of 500 to 2000 KHz), thus may have to search for signals. This search is complicated by "foldover" peaks, which are artifacts of the FT method when peaks fall outside the spectral window.
7-MULTI-3.4 Relaxation Issues
Relaxation mechanisms are often different than those seen for H and C, which are principally Dipole-Dipole (DD) (see Sect. 8-1). In particular, chemical shift anisotropy relaxation (CSA) becomes important for many nuclei, especially at high field, even to the point of causing significant line broadening. There may, therefore, be little or no NOE signal enhancement when proton-decoupling is applied (see Sect. 8-2).
If the nucleus has a negative gyromagnetic ratio (15N, 119Sn, 29Si, 125Te and a few others) nulled or negative signals can be observed under conditions of proton decoupling because the NOE is negative and can cancel or overwhelm the normal absorption signal (see Fig. 7.3.6). For these nuclei it may be necessary to use polarization transfer techniques.
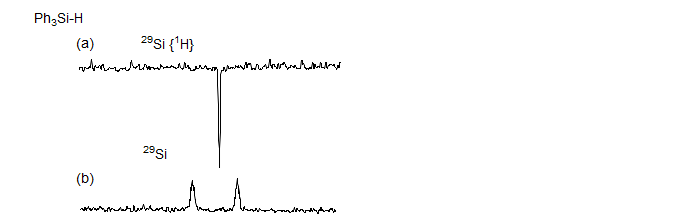
Figure 7.3.6. Proton-decoupled (a) and coupled (b) 29Si spectra of Ph3SiH, illustrating the negative NOE seen for the silicon signal (R. K. Harris, B. J. Kimber, Appl. Spectrosc. Rev., 1975, 10, 117)
Next Section: 7.4 - Quadrupolar nuclei · Previous Section: 7.2 - Effects on H, C NMR · Home