7-MULTI-4 NMR Spectroscopy of Quadrupolar Nuclei (Spin > ½)
7-Multi-4.1 Fundamentals
The principal difficulty with the NMR spectroscopy of quadrupolar nuclei is that the spin-lattice relaxation time (T1) can be very short, and broad lines (or none) are observed. T1 is determined by two factors:
(a) The electric quadrupole moment (Q). A large moment can result in efficient relaxation of the nucleus by molecular motion, hence very broad lines. Thus 125I- in water (I = 5/2, Q = 0.6) has ν½ of 1800 Hz. All other iodo compounds are much less symmmetric and have lines so broad their NMR signals cannot be detected. Deuterium (I = 1, Q = 0.0028) and 6Li (I = 1, Q = 0.00046) have among the smallest electric quadrupole moments of all isotopes and are easy to observe and usually give lines sharp enough to resolve J coupling.
(b) The presence of electric field gradients across the nucleus. Molecules with high symmetry (tetrahedral and octahedral) in which the quadrupolar nucleus in question is at the center of symmetry can give sharp lines and J-multiplets.
14NH4+ T1 >50 sec, line width <0.1 Hz.
CH3-C14N T1 = 0.022 sec, line width >50 Hz.
The number Q can be thought of as the coefficient of friction between molecular motion (rotation of the electric field of the molecule) and the nuclear spin. A high value of Q means that the nucleus is very asymmetric (oblate or prolate spheroid charge distribution) and interacts strongly with a non-spherical charge distribution in the molecule, so that molecular tumbling causes efficient nuclear spin reorientation. If the nucleus has spherical charge distribution (i.e. if I = ½) then there is no friction; conversely if the molecule has spherical charge distribution (i.e. tetrahedral or octahedral symmetry) then there is also no friction, even if I > ½.
The effect of symmetry is illustrated in Figs. 7.4.1 and 7.4.2. In the 14N NMR spectra in Fig. 7.4.1 the tetrahedral ammonium ion gives sharp peaks, whereas ammonia and unsymmetrical ammonium ions give broadened, partially resolved or unresolved signals.For other 14N and 15N spectra see: 1, 2.
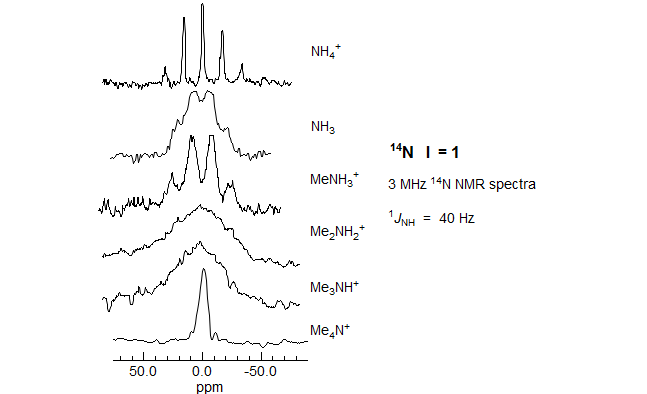
Figure 7.4.1. 3 MHz 14N NMR spectra (I = 1, Q = 0.016) (Ogg, R. A.; Ray, J. D. J. Chem. Phys. 1957, 26, 1339, from Emsley, Feeney, Sutcliffe, Vol 2. p. 1038). The ammonia sample was neat, the others were aqueous solutions. All are on the same horizontal scale, but chemical shifts are widely different.
The octahedral aluminum (Fig. 7.4.2) coordinated to six trimethyl phosphate ligands gives a sharp multiplet due to JP-Al in both the 27Al and 31P spectra, whereas the one coordinated to five phosphates and one water gives a broad signal because there is now an electric field gradient at the aluminum.

Figure 7.4.2. 27Al (I = 5/2, Q = 0.15 1024 cm-1, 22.63 MHz) and 31P (24.288 MHz) NMR spectra of a solution Al(ClO4)3 containing excess trimethyl phosphate in nitromethane (left two spectra) and aqueous nitromethane (right spectrum) (from J.-J. Delpuech et al. J. Am. Chem. Soc. 1975, 97, 3373).

Figure 7.4.3. A multinuclear NMR (AM 360, 3/2 THF/ether, -127 °C) study of a lithium reagent (courtesy Bob Dykstra). All spectra are proton decoupled. The C-Li carbon was 100% enriched with 13C, this allowed observation of coupling between C and each of the nuclei connected to it (Si, H, Se and Li). The 7Li (I = 3/2, Q = 0.02) shows normal multiplet structure due to coupling with 13C of the carbanion and 31P of the HMPA (O=P(NMe2)3) ligands.
7-Multi-4.2 Summary of Coupling in Multinuclear NMR
· Two spin ½ 100% abundant nuclei (1H, 19F, 31P, 103Rh, 89Y)
Both nuclei will show full coupling, just as in proton-proton coupling
· One spin ½ 100% abundant nucleus and one low abundance nucleus.
The low abundance nucleus will show full coupling, the 100% abundant nucleus will show satellites.
· Two spin ½ low abundance nuclei.
Both nuclei will show satellites. Homonuclear coupling only seen as satellites.

· One spin ½ nucleus and one quadrupolar nucleus.
The appearance of both signals will depend on the the T1 of the quadrupolar nucleus
(we can assume that the spin ½ nucleus will always have relatively long T1).

Exercise: Analyze and interpret ALL of the peaks in the proton NMR spectrum of formamide below:

Figure 7.4.4. 500 MHz 1H NMR spectrum of formamide in CD3CN (Ferris, T. D.; Lee, P. T.; Farrar, T. C MRC, 1997, 35, 571)
Solution Structures of Tributylstannyllithium

Figure 7.4.5 "Trimethylstannyl- and Tributylstannyllithium: Solution Structure in Ether, THF and HMPA," Reich, Borst, Dykstra, Organometallics 1994, 13, 1.
Structure of PhLi in Ethereal Solvents

Figure 7.4.6. 13C NMR spectra of 0.08 M Ph6Li in THF at -111 °C. PhLi is a mixture of monomer and 4-center dimer (Reich, Green, Medina, Goldenberg, Gubmundsson, Dykstra, Phillips, J. Am. Chem. Soc. 1998, 120, 7201-7210)

Figure 7.4.7. 13C NMR spectra of 0.16 M Ph6Li in ether at -106 °C. In this solvent PhLi is a mixture of 4-center dimer and cubic tetramer.
Exercise: R-94Q. Identify all peaks in the spectra below, identify and estimate the size of all coupling constants. The middle spectrum B is of a mixture of the two homo-dimers and the hetero-dimer. The 13C NMR spectra shows the P-Ph signals only (not the Se-Ph ones). The spectra were taken on a 360 MHz (1H) NMR spectrometer (Reich, H. J.; Dykstra, R. R. Organometallics 1994, 13, 4578).

Previous Section: 7.3 - Spin 1/2 Nuclei · Home