8-TECH-4 Saturation Transfer and EXSY
If two nuclei with different chemical shifts are undergoing a chemical exchange, and the first is saturated with the decoupler, then the second will also become partially saturated as the nuclei move back and forth between the two sites. This process is known as Saturation Transfer (sometimes referred to as the Forsen Experiment). It allows the measurement of rates that are somewhat slower than those measurable by DNMR - rate constants of the order of k = 1/T1 can be determined by saturation transfer. Below are some spectra which illustrate the saturation transfer technique as applied to cyclohexane ring inversion using a continuous wave spectrometer. The inversion can also be studied by DNMR techniques (Anet, Bourn J. Am. Chem. Soc. 1967, 89, 760). The spectra are of cyclohexane-d11, with deuterium decoupling.
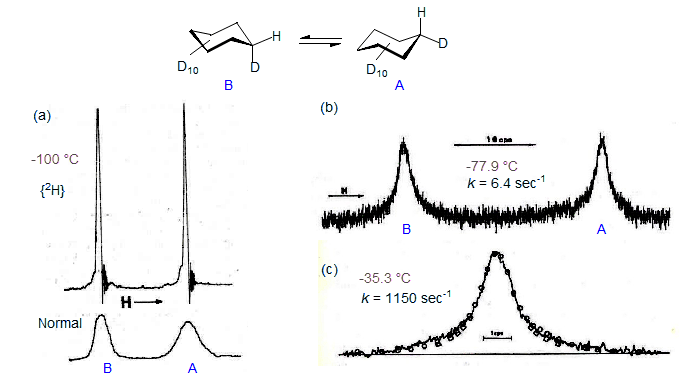
Figure 8.4-1. 60 MHz NMR spectra of C5D11H, illustrating DNMR spectra under (a) conditions of slow exchange with and without deuterium decoupling, (b) below coalescence and (c) above coalescence. The circles in spectrum (c) represent calculated line positions for k = 1150 sec-1
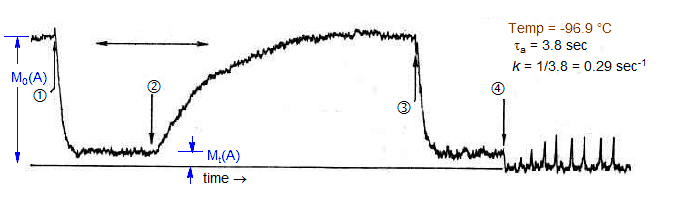
In the graphic below a Forsen saturation transfer experiment is being performed. At (1) a saturating radiofrequency was applied to the downfield signal B, and the intensity of the upfield signal A was monitored. At (2) the RF was turned off and the magnetizations were allowed to recover. At (3) the RF is turned on again. At (4) the recovery of the downfield peak was monitored by sweeping across it repeatedly.
In the trace below, the same experiment is performed at -105.3 °C. Inversion is slower here, and so the decay of magnetization is slower after (1), and the equilibrium magnetization at (2) of the upfield peak A is higher.
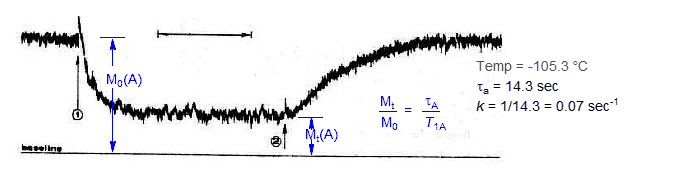
The equilibrium magnetization (Mt/M0) at (2) in each experiment is given by the ratio τA/T1A. If T1 is known (it is about 25 sec at this temperature) then the lifetime of the upfield signal can be calculated, and the rate constant determined (k = 1/τA). The rate constant can also be determined from rate of loss of magnetization of A after (1).
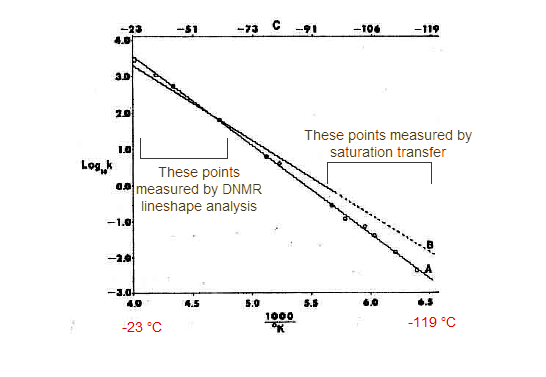
These experiments, combined with DNMR lineshape experiments at higher temperatures, allowed a very accurate measurement of the activation parameters for cyclohexane ring inversion. The graph below shows the results (line A), as well as the results of an earlier study using DNMR alone (dotted line B).
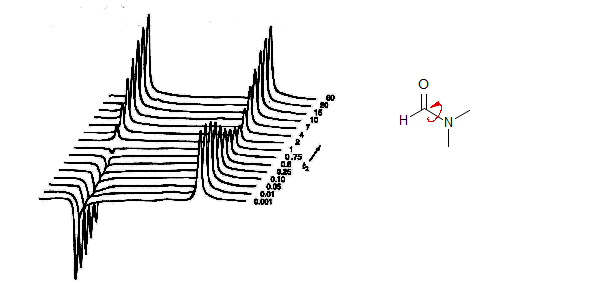
A different method for doing this experiment using an FT spectrometer is shown below. A soft (selective) 180° 1H pulse is applied to the downfield methyl group of N,N-dimethylformamide, and the magnetization is followed as the rotation around the C-N bond transfers the magnetization from the downfield pulsed Me group to the upfield one. Both magnetizations recover as T1 relaxation restores the equilibrium population differences (from Roberts, ABCs of FT-NMR, p. 280).
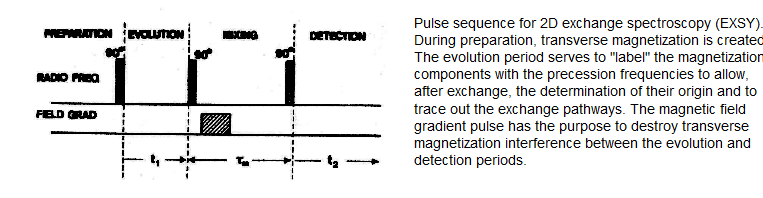
A two dimensional version of the Forsen experiment is the EXSY experiment (EXchange SpectroscopY, Perrin, C. L.; Dwyer, T. Chem. Rev. 1990, 90, 935). This experiment allows much more precision in establishing exchange pathways in mechanistically complex processes than does the 1D Forsen experiment.
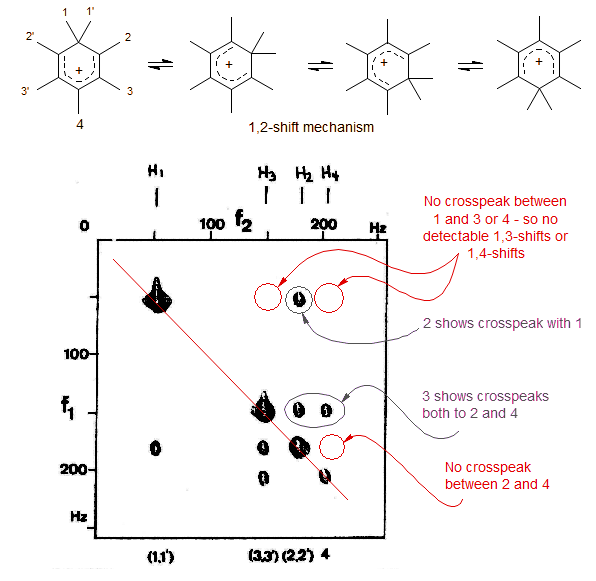
An early example of an EXSY experiment is the methyl migration in the heptamethylbenzenonium ion (Meier, B. H.; Ernst, R. R. J. Am. Chem. Soc. 1979, 101, 6441). The 1,2-methyl shift mechanism is unambiguously proven by the observation of cross peaks only for 1/2, 2/3 and 3/4 methyl groups. The absence of cross peaks between 1,3- , 1,4- and 2,4-related methyl groups shows that only 1,2-shifts, and no 1,3 or 1,4 shifts occur.