8-TECH-9 Two Dimensional NMR
In 2- (or multi-) dimensional NMR spectroscopy multipulse sequences are employed to provide additional information not obtainable (or not easily obtainable) from one-dimensional spectra. The most useful and commonly used forms of 2D NMR spectroscopy provide correlations between proton (or other NMR-active nuclei) signals based on some interaction between them, most frequently J-coupling (H-H. H-C or even C-C), but it could also be dipolar coupling (the NOE interaction), rates of chemical exchange (DNMR swapping of spin environments) or relative diffusion rates (DOSY and related experiments).
The most common types of 2D experiments are listed below.
COSY: Homonuclear correlated spectroscopy. Correlation between protons that are coupled to each other. (Bax J. Magn. Reson. 1981, 42, 169). There are many modified version of the basic COSY experiment: DQF-COSY (double-quantum filtered), COSY45, LRCOSY, ECOSY.
TOCSY: Total Correlation Spectroscopy (also referred to as the HOHAHA experiment). Uses a spin-lock for coherence transfer. During the spin-lock all protons of a coupled system become "strongly coupled," leading to cross peaks between all resonances of a coupled system. Has some advantages over COSY in that small couplings can be detected (in COSY small couplings give very weak cross-peaks because the peaks are antiphase).
CH-COSY or HETCOR: Heteronuclear correlation, usually between 1H and 13C resonances mediated by JC-H. The experiment can be run using either 1JC-H or longer range couplings. It has poor sensitivity because the observed nucleus is 13C, and has been largely replaced by the inverse detection experiments HMBC and HMQC. (Review: Martin, Zektzer Magn. Reson. Chem. 1988, 26, 631). For examples see: Lankin J. Org. Chem. 1990, 55, 5139.
HMQC: Proton detected heteronuclear multiquantum coherence. Similar to CH-COSY or HETCOR experiment, except that the inverse detection using a DEPT sequence provides much better sensitivity (Bax J. Am. Chem. Soc. 1986, 108, 4285). Used to correlate proton and carbon signals using either one bond or longer range couplings. For examples see Bashkin J. Org. Chem. 1990, 55, 5125.
HSQC: Heteronuclear Single Quantum Coherence. This is a CH correlation experiment which uses proton detection of the 13C signals using an INEPT sequence. It shows higher resolution in the C-dimension than does the related HMQC experiment.
HMBC: Heteronuclear Multi-Bond Connectivity. This experiment is a lot like HMQC, but it is optimized to detect proton-carbon correlation over 2 and/or 3 bonds (Bax, J. Am. Chem. Soc. 1986, 108, 2093). Although the sensitivity is better than direct detection methods, it is still not very good, since the magnetization has to be transferred twice, first from proton to carbon and then back to proton via weak long-range interactions, which require relatively long delay times and thus much opportunity for loss of signal intensity by T1 and T2 relaxation. For a modification to distinguish 2 from 3-bond coupling see: Griesinger, Tetrahedron Lett. 1996, 37, 363.
EXSY: Exchange Spectroscopy - the 2D equivalent of the Forsen saturation transfer experiment. The transfer of magnetization, however, is due to chemical exchange between A and B, rather than an NOE. EXSY uses an identical pulse sequence to the NOESY experiment, and is thus subject to interference by NOE. The magnetization transfer will be the equivalent of a negative NOE (Perrin, C. L.; Dwyer, T. Chem. Rev. 1990, 90, 935).
NOESY, ROESY: Proton-proton correlation mediated by dipolar coupling (NOE effect). Correlation between protons that are close in space. This is the single most powerful NMR technique for determining the 3-dimensional structure of molecules - from conformations and configurations of small molecules to the 3-dimensional structure of small proteins.
HOESY: Heteronuclear Overhauser Effect Spectroscopy. Correlation between protons and heteronuclei that are close in space. Requires that the heteronucleus be relaxed significantly by dipole-dipole interactions with nearby protons or other magnetic nuclei, and so fails with nuclei relaxed exclusively by chemical shift anisotropy or quadrupolar mechanisms. Used extensively in the spectroscopy of organolithium reagents, since 6Li works well in this experiment.
INADEQUATE: Incredible Natural Abundance Double Quantum Transfer Experiment. 2-Dimensional JC-C This double quantum coherence experiment produces a C-C correlation using 1JCC. It is perhaps the most powerful structure-determination tool of all, but suffers from very low sensitivity (Bax, A.; Freeman, R.; Frenkiel, T. A.; Levitt, M. H. J. Magn. Res. 1981, 43, 478)
DOSY, PGSE: Diffusion-Ordered SpectroscopY, Pulsed Gradient Spin-Echo. In these experiments molecular diffusion is detected by applying a pulse gradient of varying strength for a set time period, and measuring the loss of signal intensity resulting from diffusion as a function of gradient strength. The method provides a way of estimating molecular weights (to the extent they relate to diffusion coefficients) of multiple NMR active species in a single solution, and is well suited to help characterize reactive species which cannot be purified or isolated.
Relationship Between Free Induction Decay and Spectrum
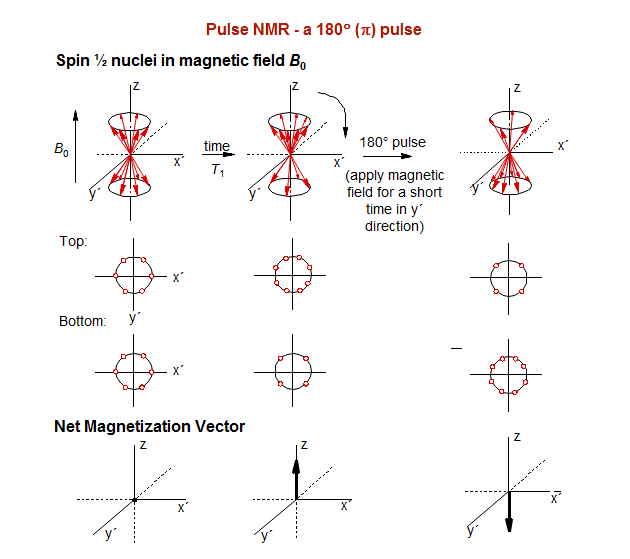
Before we can discuss multi-pulse NMR experiments (including 2D experiments) we have to review the basics of FT NMR. The simplest NMR experiment is a 90° (π/2) pulse, followed by measurement of the Free Induction Decay (FID, magnetization in the y' direction of the rotating frame). The magnetization will rotate around the Z axis at a rate of 1/Δν, where Δν is the difference in frequency between the spectrometer pulse, and the Larmor precession of the nucleus.
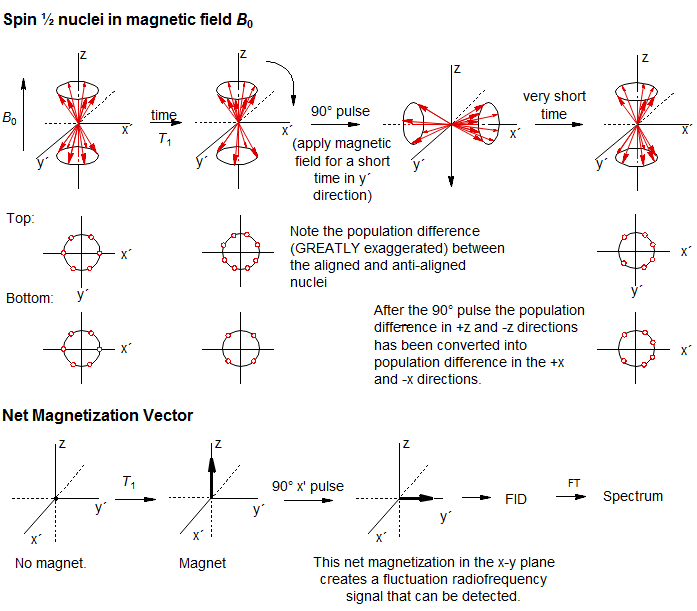
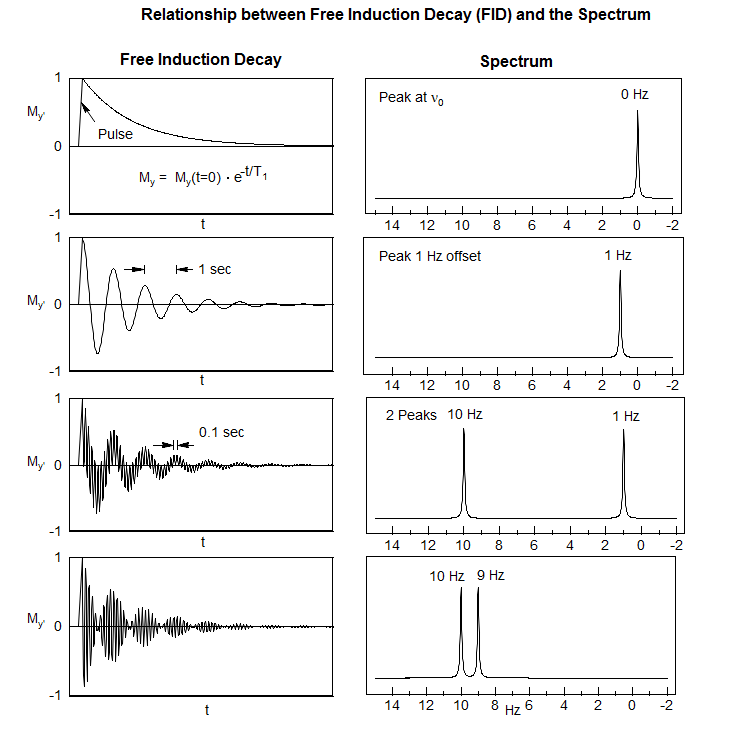
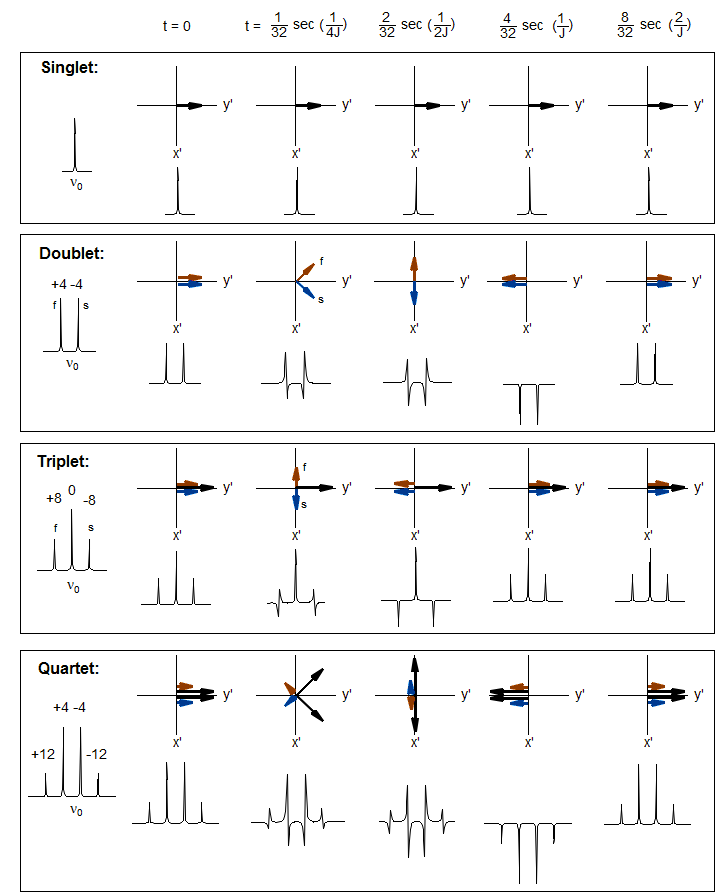
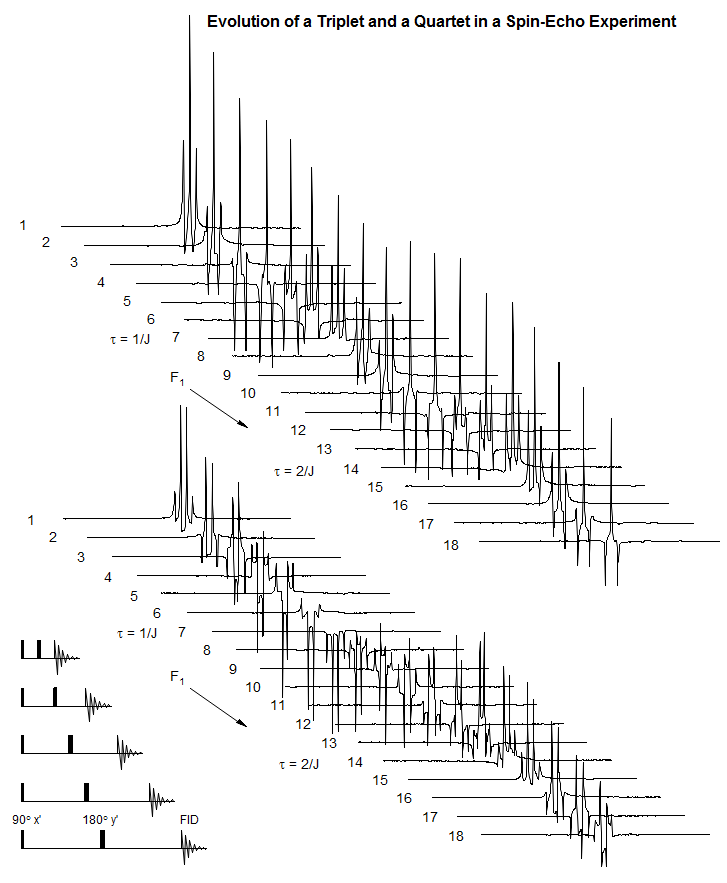
Among the most important multi-pulse experiments are those in which correlations or distinctions between chemical shifts and couplings are made. On the following page are depicted the evolution of magnetization of a series of multiplets as a function of time, and the expected appearance of the spectrum if an FID was collected at the end of the time period, and FT'd.
Note the different behavior of the multiplets, particularly at a delay time of 1/J.
Time Evolution of Magnetization Vectors Signals
The Spin-Echo Pulse Sequence for Distinguishing Couplings and Chemical Shifts
The spin-echo sequence, in which a 180° pulse is placed exactly in the middle of a delay period t1, is central to most multi-pulse NMR experiments. In addition to refocusing magnetic field inhomogeneities, it refocuses chemical shifts, so that at the end of the delay period all magnetization which was initially in the y' direction will again be in the y' direction, UNLESS the signal was part of a homonuclear spin system. In the latter case, the signals will not refocus, but continue to evolve throughout the delay period. This is because the 180° pulse will also invert the coupling partner (α to β, β to α) so that the population which was "slow" before the refocussing pulse becomes "fast" after, and vice versa. There is thus a fundamental difference between the behavior of a chemical shift and a coupled multiplet.
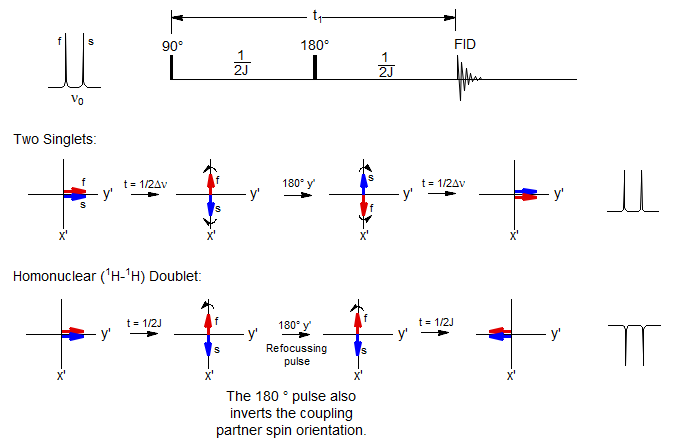
As illustrated above, if the total delay period t1 is 1/Δν for the chemically shifted pair of peaks, or 1/J for the doublet, then the chemical shifted peaks will be positive when the FID is collected, and the doublet negative. This behavior is fundamental for all 1D and 2D experiments which involve the manipulation and detection of couplings.
If the doublet is due to a heteronuclear coupling (AX), then the A doublet will behave identically to the chemically shifted peaks, since the required inversion of the spin of the coupling partner X will not have happened. Such experiments can still be done, however. All that is required is a simultaneous 180° pulse on the X nucleus, as illustrated for the APT experiment described below.
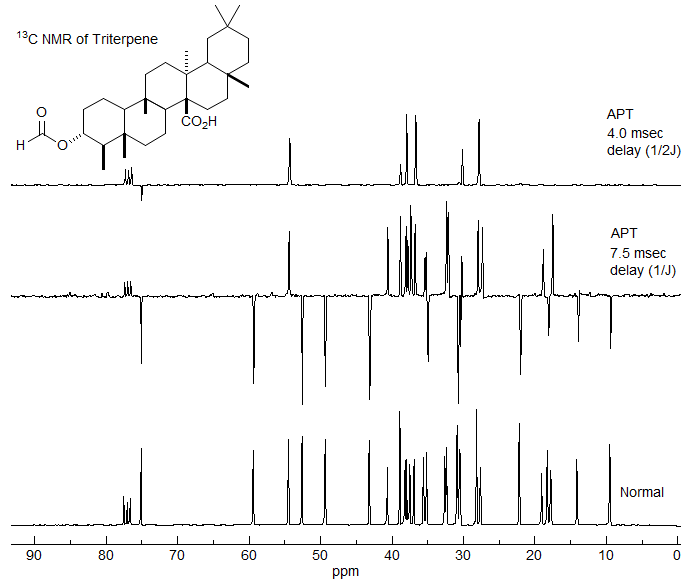
The Attached Proton Test - Distinguishing Couplings and Chemical Shifts
The Attached Proton Test (APT) experiment takes advantage of the different effects of a pulse delay and a refocusing pulse on peaks separated by a chemical shift and those separated by a coupling constant. Here we are taking a 13C NMR spectrum using a spin-echo sequence that gives either positive or negative peaks depending on whether there are an even or odd number of hydrogens on the carbon.
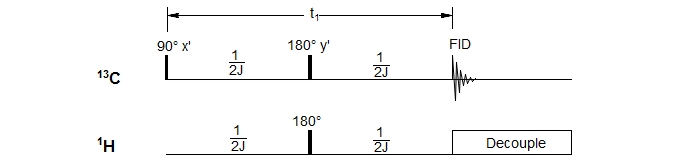
In contrast to the homonuclear spin-echo sequence, where both the observed proton and its coupling partner are inverted, in the heteronuclear version only the observed nucleus (in this case 13C) is inverted by the 180° pulse, so a simultaneous 180° pulse has to be applied to the protons to make sure that only chemical shifts are refocused and C-H couplings continue to evolve. In addition, the decoupler is turned on during collection of the FID, so C-H couplings are not observed in the spectrum.
The most useful delay is t1 = 1/J, when C and CH2 peaks are positive, CH and CH3 peaks are negative. See the Figure below for a graphic depiction of the behavior of signal intensity as a function of the delay period t1.
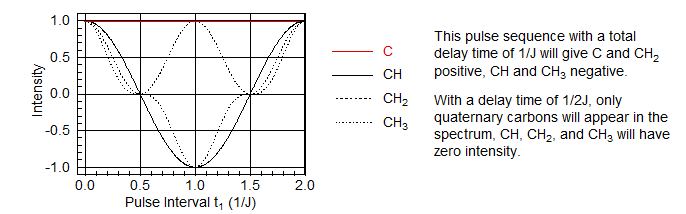
Reference to the Figure on time evolution of multiplet signals shows that at a pulse delay of 0.5/J only the singlet (quaternary carbon) has net magnetization in the x direction, all others have either offsetting x and -x vectors (CH2), or magnetization only in the y/-y direction (CH, CH3). Thus this experiment will give a quaternary carbon-only spectrum. More precisely, this experiment will suppress all signals with J = 2t1, where t1 is the total delay. If there are C-H couplings significantly different than 2t1 (as would be the case for a C≡C-H proton), then C signals may be only partially, or not at all suppressed.
This experiment has been largely replaced by the DEPT pulse sequence, which gives similar information, but with much better signal to noise, since the sequence includes polarization transfer from proton to carbon. The DEPT sequence cannot be understood in terms of vector diagrams of the sort used here.
A simple APT experiment. The bottom spectrum is a normal acquisition of a 13C NMR spectrum. In the middle spectrum a delay time t1 of 1/J has been applied, leading to positive C and CH2 signals, and negative CH and CH3 signals. In the top spectrum a delay of 0.5/J, to give a spectrum showing only quaternary carbons.
8-TECH-9.2 The 2DJ Experiment - Chemical Shifts and Couplings on Perpendicular Axes
The 2DJ experiment is not used very often, but it is one of the simplest 2D NMR experiments to understand. For the homonuclear case, the pulse sequence is:
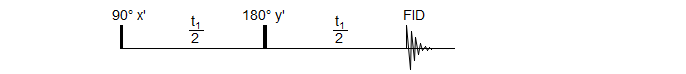
After this sequence, all signals which have only a chemical shift (singlets, the central peaks of triplets and quintets, etc) will be refocused in the y' direction, and measurement of the FID followed by Fourier transformation (FT) will result in a properly phased positive peak. Any peak which is offset due to a coupling will not be refocused, and will have different phase depending on the delay time t1.
Below are several spin-echo NMR spectra of alanylglycine, showing the phase modulation as a function of delay time.
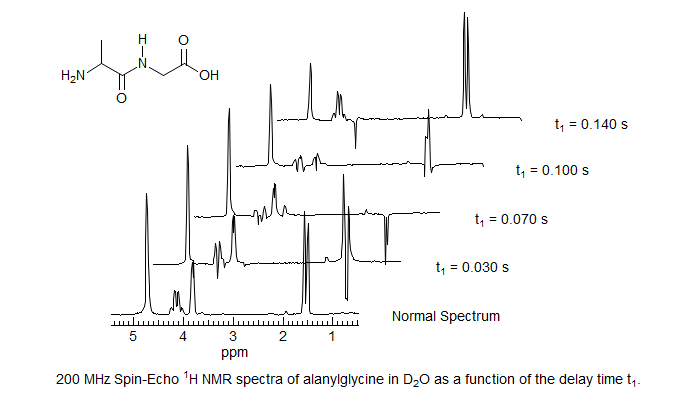
A series of spectra are taken where the variable delay t1 is uniformly incremented, as shown below. Any peak which is offset by a coupling will be phase modulated at the frequency offset from the center peak (follow the tips of the two outer lines of the triplet).
These spectra are then Fourier Transformed in the F1 dimension by taking one point from each spectrum. Signals which have no coupling component, such as the central peak of the triplet, will not be phase modulated, and will appear at the origin (0 Hz offset). Signals which have a coupling component will be phase modulated, and will be offset by the appropriate frequency (0.5 J for doublets, 1.0 J for the outer peaks of triplets). The FT process can distinguish positive and negative frequencies since the dispersion-mode component of the signals is phased in opposite directions.
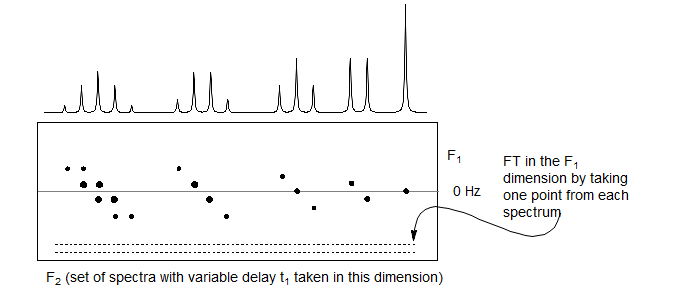
The two-dimensional spectrum that results has the coupling "tilted" by 45° from the original axis. A mathematical 45° rotation can be performed to place the coupling and chemical shift axes perpendicular to each other.
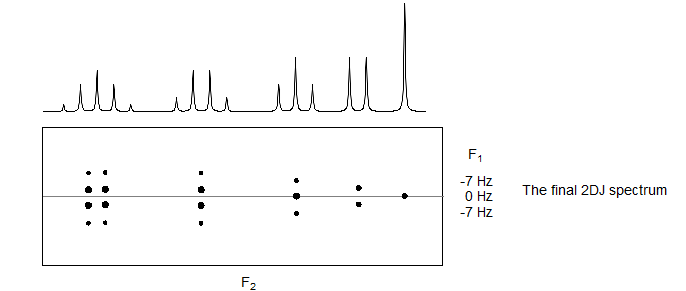
We now can take slices in the F1 dimension, to obtain individual multiplets, or a projection in the F2 dimension, to obtain a proton-decoupled proton NMR spectrum, i.e., a spectrum with only singlets. In our hypothetical example this provides the interesting insight that the apparent quintet at the left is actually two overlapping quartets. In a complicated spectrum in which multiplets may overlap badly, the experiment can lead to greatly improved analysis of the spectrum.
A 2DJ spectrum of a steroid. Almost all of the protons are resolved and give more or less first order multiplets.
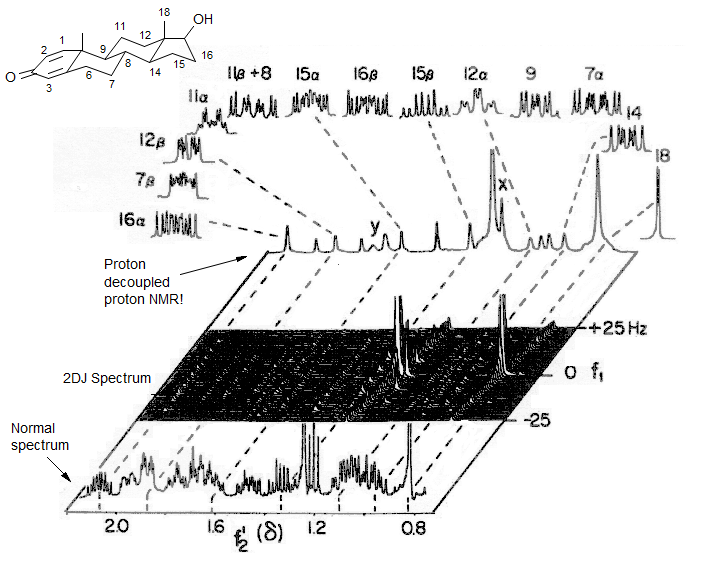
400 MHz 2DJ spectrum (X is added ethanol CH3, Y denotes the second order transitions of the 11α, 11β CH2 protons). Hall, Sanders J. Am. Chem. Soc. 1980, 102, 5708
Z-Filtered 2DJ spectrum of 4-androstene-3,17-dione at 400 MHz (Simova, Sengstschmid, Freeman J. Magn. Reson. 1997, 124, 104)
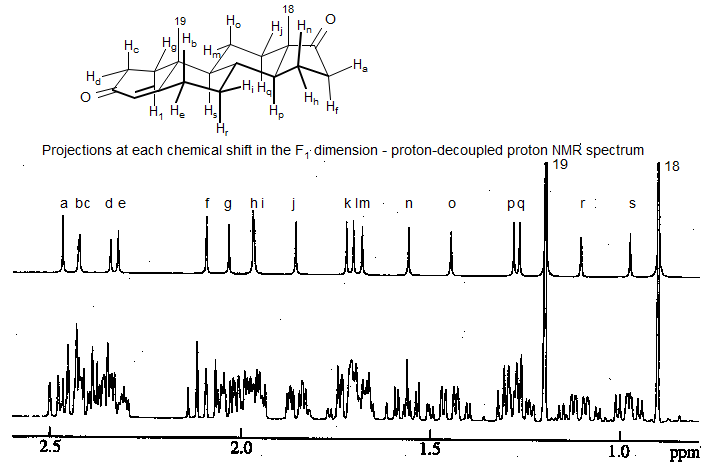
Slices of the 2DJ spectrum at each proton chemical shift:
8-TECH-9.3 The COSY Experiment - Proton-Proton Coupling Correlation
The COSY experiment is probably the most useful and most used of all 2D NMR experiments, since it provides easily accessible information about coupling pathways. The pulse sequence in its simplest form is shown below (there are many variants with additional pulses for achieving higher quality correlations and removing artifacts). Examples: 1, 2, 3.
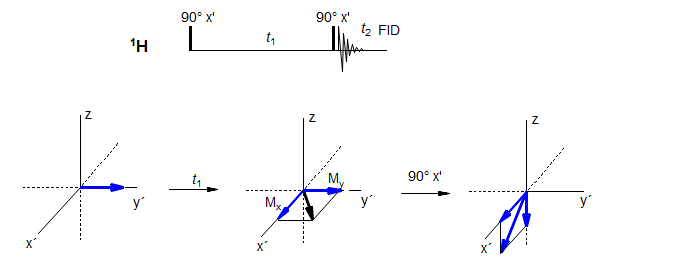
During the t1 period each of the signals is labeled by the chemical shifts and couplings. After the second 90° pulse the component of the magnetization which used to be in the y' direction is now in the z-direction, and becomes undetectable. Only the component in the x' direction evolves and is detected during collection of the FID. What cannot be shown in vector diagrams is that during t2 period the magnetization in the x-y plane contains components from both the nucleus observed as well from the frequency of its coupling partner, mediated by the coupling between them.
When the spectra are FT'd in the t1 dimension, the normal coherence is detected, such signals appear along the diagonal of the resulting 2D spectrum. In addition the coherence from the frequency of the coupled partner is also detected, and results in cross peaks at that position.
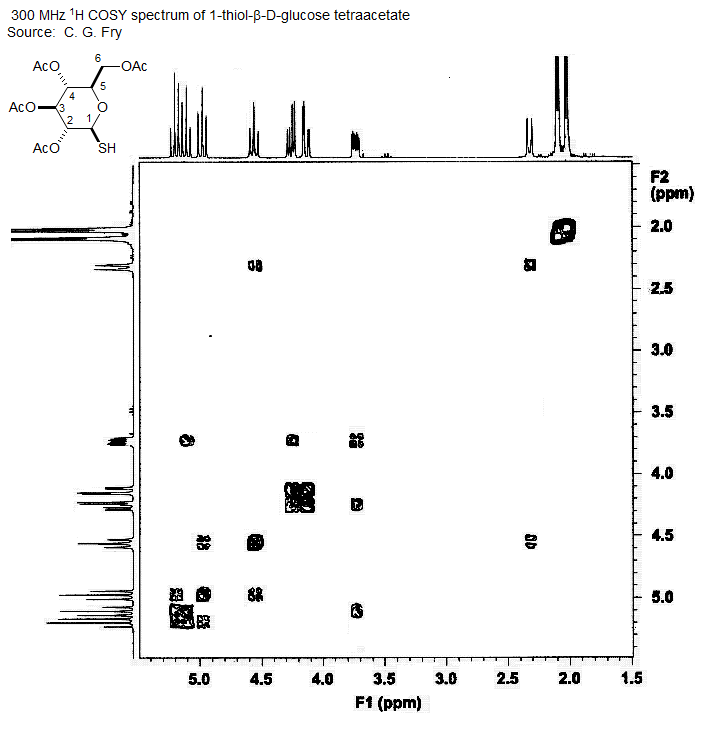
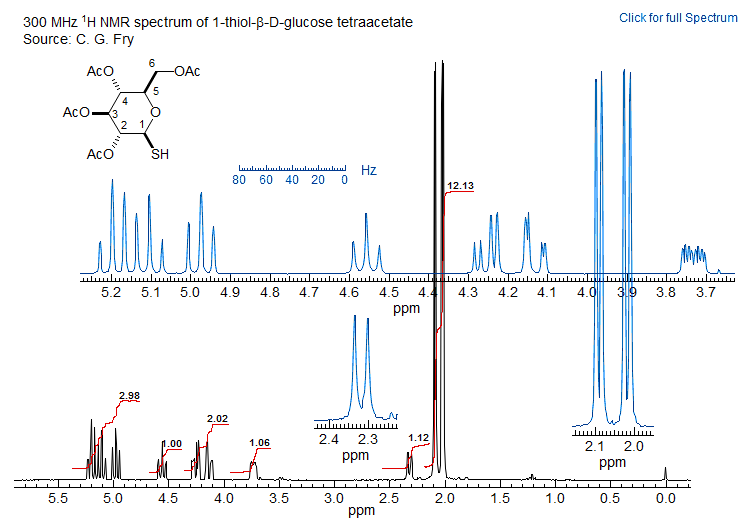
Below is shown the COSY spectrum of a thio sugar. The signals can be easily assigned, provide one can find a signal whose chemical shift or coupling pattern makes it unique to allow a firm assignment. Suitable signals are the SH proton at δ 2.3 (coupled only to one other) or the H5 proton at δ 3.7 (coupled to three other protons, although in this spectrum the smallest of the couplings is not seen, a common problem with the COSY experiment). On the following page is the 1D spectrum of the same sugar. The signals can mostly be assigned, but even a careful consideration of leaning leaves some ambiguities in the assignment.
8-TECH-9.4 The HETCOR Experiment - Proton-Carbon Correlation
Next to the proton-proton COSY experiment, probably the next most useful correlation experiments for organic chemists are those which correlate proton-carbon signals. Examples: 1.
For heteronuclear correlation experiments it is desirable to decouple the proton signals during the acquisition period. Thus the carbon signals need to be in phase at the time the decoupler is turned on, else the signal will be reduced or lost altogether. This means that a pulse sequence considerably more complicated than the simplest H-H COSY pulse sequences is required. One is illustrated below:
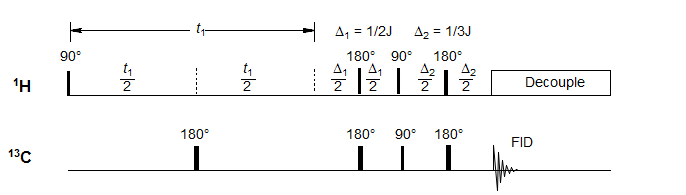
During the variable t1 period chemical shifts evolve, but the effect of coupling is removed by the 180° C pulse in the middle of the period. Thus at the end of t1 the signal contains no coupling information. The first constant delay period Δ1 (with a spin echo in the middle to refocus chemical shifts) allows the proton signals to get 180° out of phase, the 90° pulses then achieve polarization transfer. The second delay Δ2 restores proper phase relationship to give absorption mode signals. The proton decoupler is then turned on, and acquisition of 13C FID started. This pulse sequence gives signals which are decoupled in both the 1H and 13C dimensions.
The HSQC, HMQC and HMBC experiments are variants of similar pulse sequences which provide proton carbon correlation over either one bond or 2-3 bonds.
An example of a HETCOR proton-carbon correlation experiment (Source: Varian XL Series NMR Spectra Collection 1, 300 MHz, 0.8 mg sample). In this experiment the quaternary carbons are not seen, since it is a polarization transfer experiment. Distinguishing among aromatic and olefinic sp2 carbons is typically difficult, since they occur in the same chemical shift range. In this experiment the vinyl C-H protons H1, H2 and H15 are easily assigned, and hence the correlated carbons can be identified.
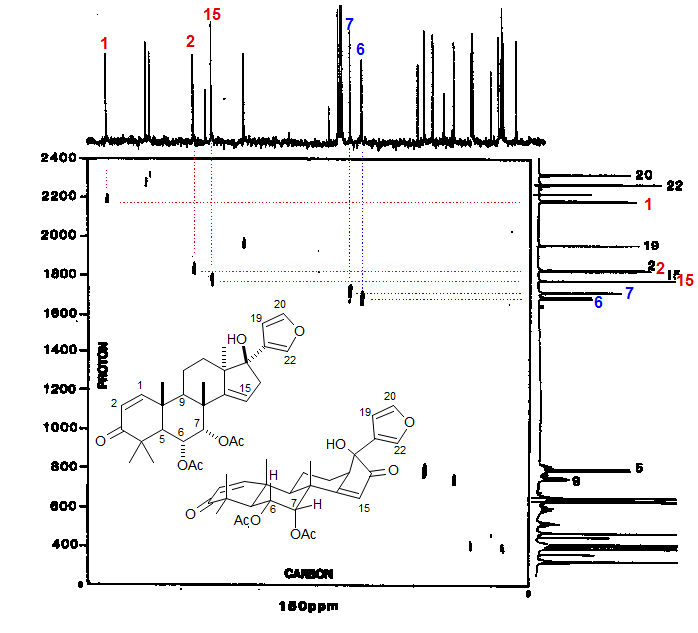
The two carbons bearing acetoxy groups (C6 and C7) can also be assigned. H6 and H7 can be distinguished because H7 is an equatorial proton, with only small vicinal couplings, whereas H6 is axial, and has one large Jax-ax coupling.
8-TECH-9.5 The NOESY Experiment - 2D Nuclear Overhauser Enhancement
The NOESY experiment detects dipolar relaxation between protons that are close in space, and as such is especially valuable in the determination of stereochemistry and conformation of complex molecules, as well as secondary structure in peptides, small proteins, oligonucleotides, carbohydrates and other complex biomolecules. The simplest pulse sequence for this experiment, which is closely related to the COSY experiment (and identical to the EXSY experiment), is shown below.
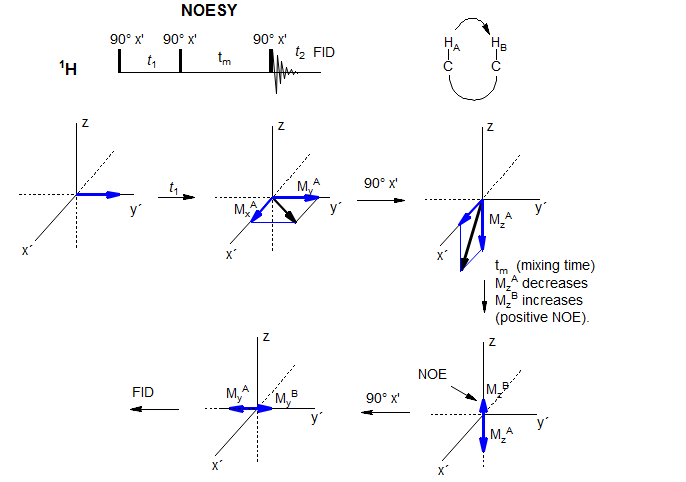
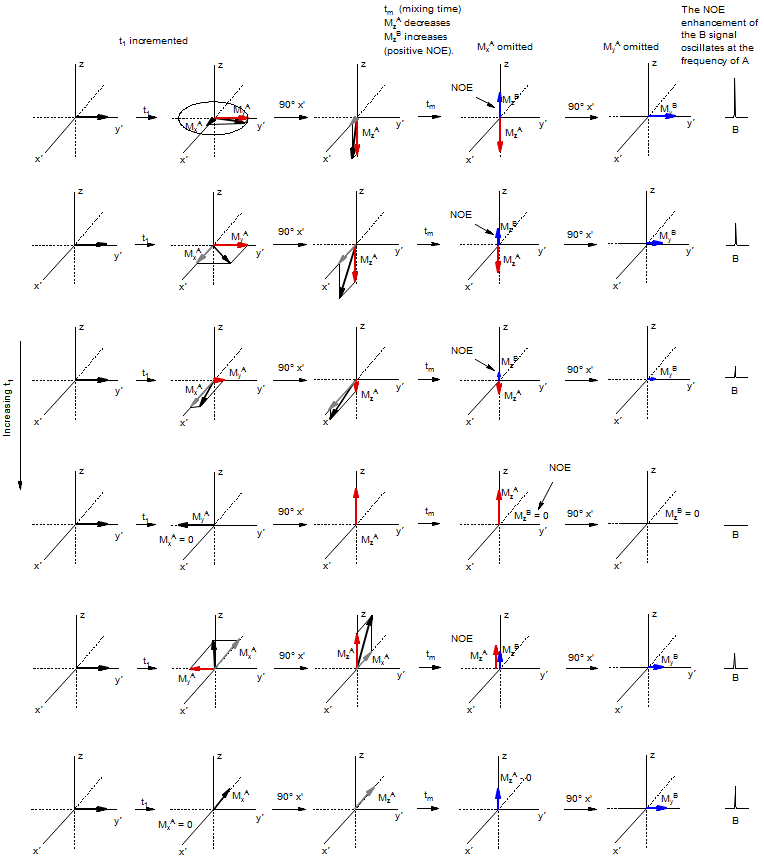
The preparation period t1 labels each of the magnetizations with the chemical shift, as MxA and MyA oscillate as a function of t1. It is the My component we are interested in here, since this will be the source of the oscillating NOE component. We will ignore the MxA component which will continue to evolve during tm, and is the source of some problems in the NOESY experiment (COSY interference).
The second 90° pulse will rotate MyA into the z-direction. This magnetization of nucleus A will cause an NOE to build up in spatially close nucleus B during the mixing time tm. Since the magnitude of the z-magnetization MzA will oscillate at the frequency of the A nucleus in the t1 dimension, the NOE magnetization at the chemical shift of B will also oscillate at this frequency. The NOE is then detected with the final 90° pulse; it will give a cross peak between A and B, since during the acquisition t2 it is rotating at νB, but its intensity is oscillating at νA.
The NOESY Experiment
Example of a NOESY experiment on a simple bicyclic lactone. Note the correlation of the vinyl proton H9 at δ 5.8 with a terminal vinyl proton, one of the OCH2 protons (H4, at δ 4.2) as well as a proton at δ 2.32. This proton has NOE interactions with one of the protons at C5 (δ 2.04) as well as one of the protons at C4 (δ 2.78). Thus this is the bridgehead proton (H8a). The NOE interaction between H9 and H8a proves that the ring is cis-fused, a typical task for which NOE experiments are well suited. The cis-fused ring stereochemistry is also supported by the NOE interaction between H1' (δ 3.96) and H5' (δ 1.74).
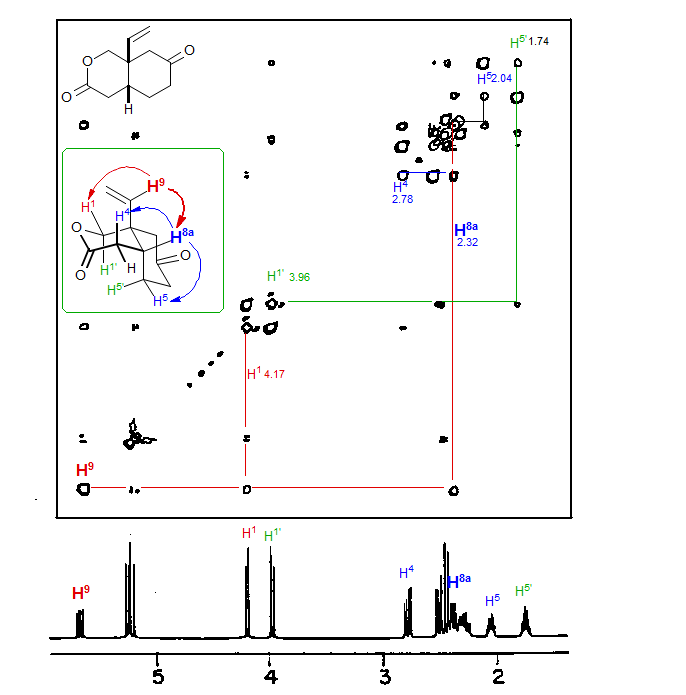
Phase sensitive NOESY spectra in CDCl3 (total time 3.7 h) (Meyerhoff, D. J. Magn. Reson. Chem. 1987, 25, 843). A relatively long mixing time (3 s) was needed to see the key NOE interaction between H8a and H9 because H9 had a considerably longer T1 relaxation time (3 s) than most of the other protons (1-2 s).
8-TECH-9.6 Carbon-Carbon Coupling - the INADEQUATE Experiment
The INADEQUATE experiment (Incredible Natural Abundance Double Quantum Transfer Experiment) is a 2D double quantum coherence experiment that allows measurement of C-C coupling at natural abundance. It is usually used to establish 1JCC connectivity, but can also be used for 2JCC or 3JCC. It is perhaps the most powerful structure-determination tool of all, but suffers from very low sensitivity, because it is detecting the tiny concentration of molecules that contain two adjacent 13C nuclei (ca 1 molecule out of 10,000, if there is no symmetry) (Bax, A.; Freeman, R.; Frenkiel, T. A.; Levitt, M. H. J. Magn. Res. 1981, 43, 478)
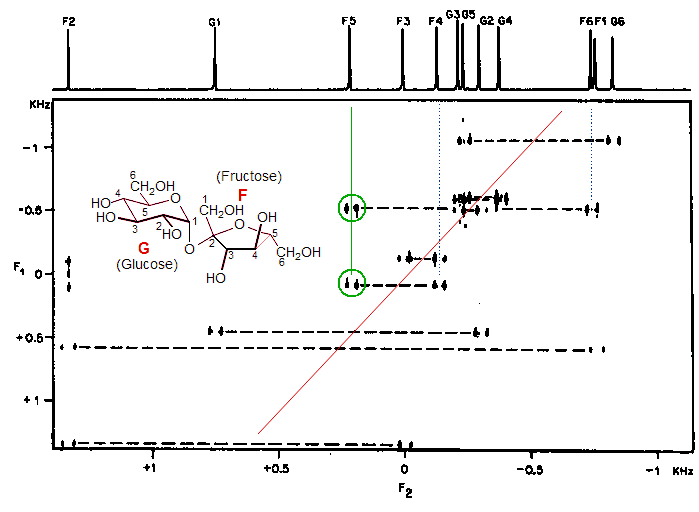
Below is shown the 2D 13C NMR spectrum of sucrose using the INADEQUATE pulse sequence. The AB or AX-type satellite spectra are the four-line patterns joined by broken horizontal guidelines, which connect carbons which are directly bonded (1J(13C-13C)). Their centers of gravity lie along a line with ΔF1/ΔF2 = 2. Note that a terminal carbon (like F1, G1, F6 or G6) shows connectivity to only one other carbon, whereas carbons in the middle of the sugar chain show connectivity to two carbons (e.g. F5 shows a C-C correlation with F4 and F6). If all cross-peaks can be detected and there are no unresolvable coincidences of chemical shift, an INADEQUATE spectrum allows determination of the complete C-C connectivity of a molecule.