[2,3]Sigmatropic Rearrangements
A large number of [2,3]sigmatropic rearrangements have been studied. They can be divided into three types, Ylidic, Anionic or Carbenic, depending on the nature of the charge relationships between the X-Y fragment. X and Y can be O, N, C, S, Se, P and others.
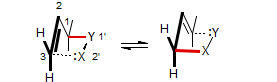
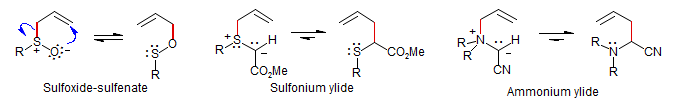
Ylidic (oxide, imide): one partner is a dipolar structure, the other is neutral. The ylids are typically prepared either by deprotonation of a sulfonium, ammonium or other allylic onium salt, or by reaction of a carbene or a carbene source with the lone pair of an allyl sulfide, amine, ether, etc. With a few important exeptions (such as the sulfoxide-sulfenate equilibrium, which is marginally on the side of the sulfoxide), these reactions strongly favor the neutral over the ylidic form.
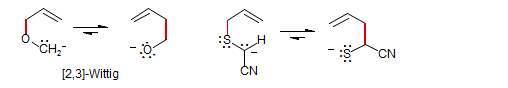
Anionic: Each partner bears a negative charge on the X or Y atom. The all-carbon system is essentially unknown (for an example see Baldwin Chem. Commun. 1970, 165) but systems where the negative charge migrates from carbon to a heteroatom like O or S are quite common:
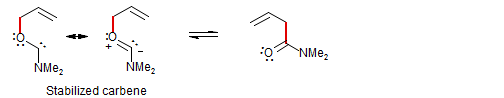
Carbenic: one partner is a carbene, the other has an X=Y double bond: