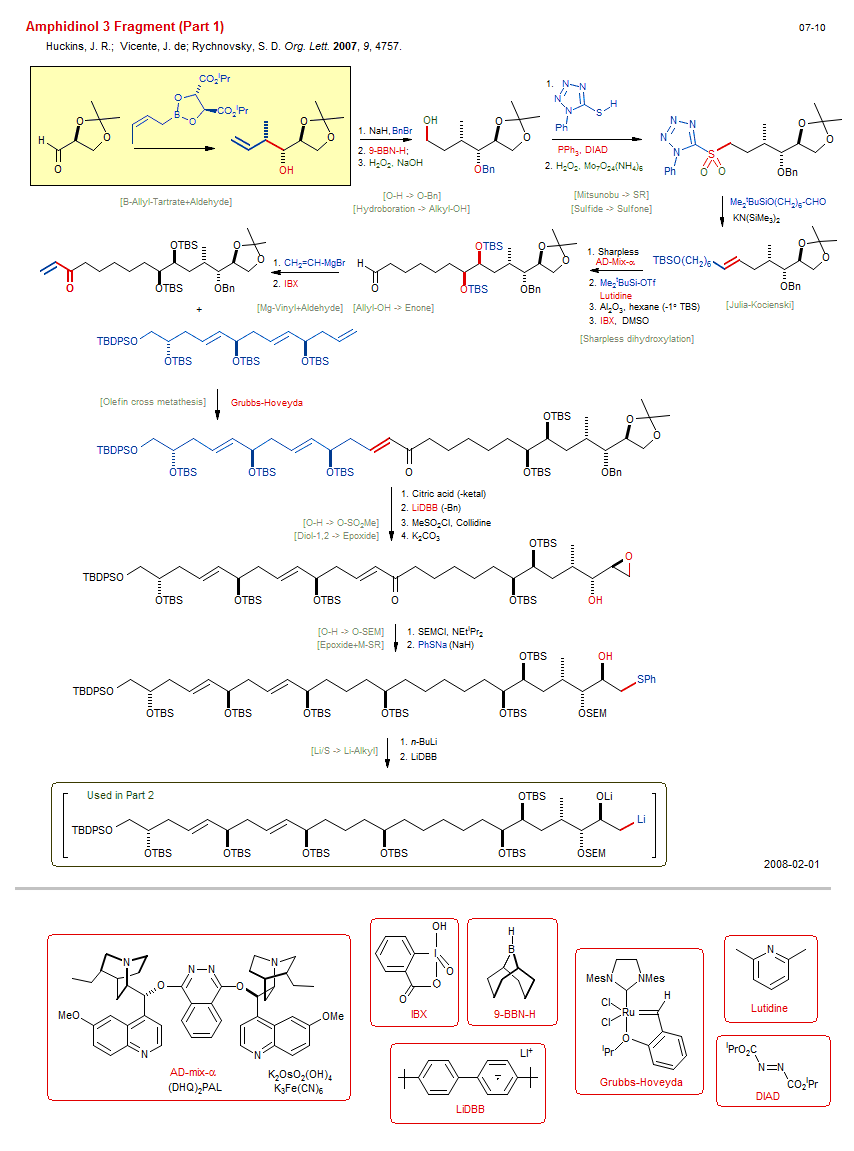
Total synthesis of Amphidinol 3 Fragment (Part 1):
| Reference: | Huckins, J. R.; Vicente, J. de; Rychnovsky, S. D. Org. Lett. 2007, 9, 4757. DOI (Part 2) |
| Reactions: | O-H → O-SiMe2tBu • O-SiMe2tBu → O-H • CompE+-Si-O/Si-O+H2O • Alkyl-OH → Aldehyde • O-Bn → O-H • B-Allyl-Tartrate+Aldehyde • Aldehyde+B-Allyl (asym) • Hydroboration → Alkyl-OH • Mitsunobu → SR • Alkyl-X → Alkyl-SR • Julia-Kocienski • Li-Sulfone+Aldehyde • Sharpless dihydroxylation • Alkene → Diol-1,2 (asym) • Olefin cross metathesis • O-H → O-SO2Me • Diol-1,2 → Epoxide • O-H → O-SEM • Epoxide+M-SR • Thiolate+Epoxide • Mg-Vinyl+Aldehyde • Allyl-OH → Enone • Sulfide → Sulfone • Li/S → Li-Alkyl • O-H → O-Bn • |
| Reagents: | Allyl-B(tartrate) • Chiral auxiliary (boronate) • NaH • BH-BBN • H2O2, NaOH • Thiol, tetrazole • PPh3, DIAD • H2O2, Mo • KN(SiMe3)2 • AD-Mix • SiMe2tBu-OTf • Lutidine • Al2O3 • IBX • Vinyl-MgX • IBX • Grubbs-Hoveyda • LiDBB • Collidine • Carbonate, potassium • NEtiPr2 • Thiolate, phenyl • DMSO (solvent) • Citric acid • Benzyl-X • BuLi(n) • LiDBB • |