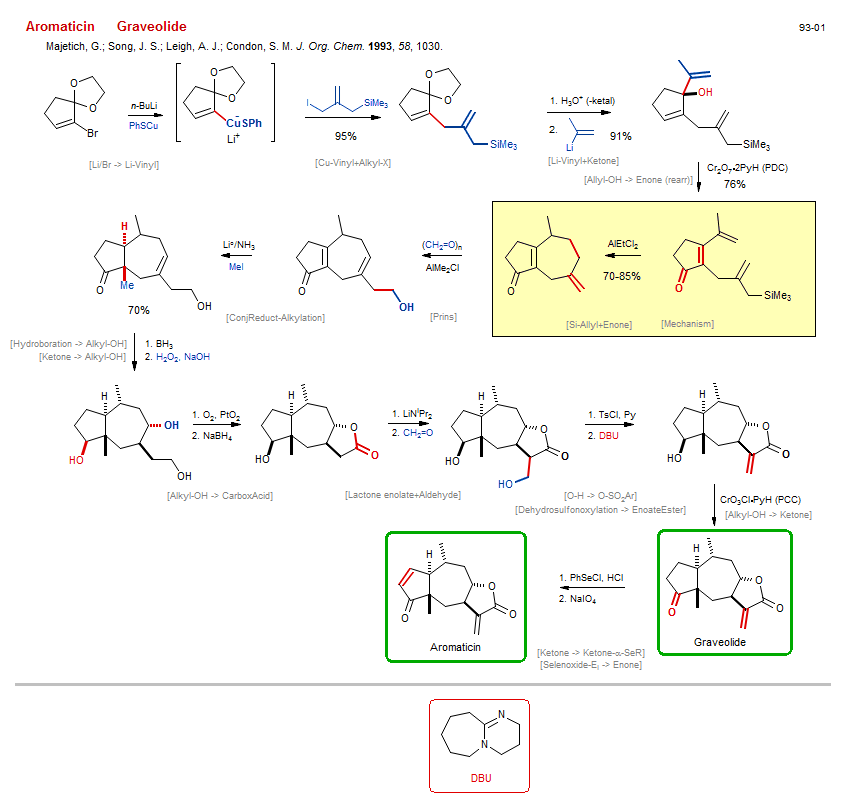
Total synthesis of Graveolide:
| Reference: | Majetich, G.; Song, J. S.; Leigh, A. J.; Condon, S. M. J. Org. Chem. 1993, 58, 1030. DOI |
| Reactions: | Ketal → Enone • Alkyl-OH → CarboxAcid • Prins • Cu-Vinyl+Alkyl-X • Allyl-X+Cu-R • Selenoxide-Ei → Enone • Ketone → Enone • Li/Br → Li-Vinyl • Hydroboration → Alkyl-OH • Li-Vinyl+Ketone • Enone+Li-R • Lactone enolate+Aldehyde • Aldol-Li → Aldol • ConjReduct-Alkylation • Ketone enolate+Me-I • Enone ConjReduct • Alkyl-OH → CarboxAcid • Lactone-5 • CompOx-Alkyl-OH/Alkyl-OH+O2/Pt • Si-Allyl+Enone • Enone+Si-R • ConjAdd Si-Allyl • Sakurai allylation • Cycloheptane • Bicyclo(520) • Mechanism • Dehydrosulfonoxylation → EnoateEster • Alkyl-OH → Ketone • Ketone → Ketone-α-SeR • Allyl-OH → Enone (rearr) • Ketone → Alkyl-OH • O-H → O-SO2Ar • CompNu-O-H/O-H+RSO2X • |
| Reagents: | BuLi(n) • CuSPh • Vinyl-Cu • Allyl-I • Allyl-SiR3 • Propenyl-Li • Cr2O7·2PyH (PDC) • Formaldehyde • AlMe2-Cl • Li°/NH3 • MeI • BH3 • O2, PtO2 • BH4-Na+ • LiNiPr2 • TsCl, Py • DBU • CrO3Cl·PyH (PCC) • SePh-Cl • Periodate, sodium • H2O2, NaOH • Formaldehyde • AlEtCl2 • |