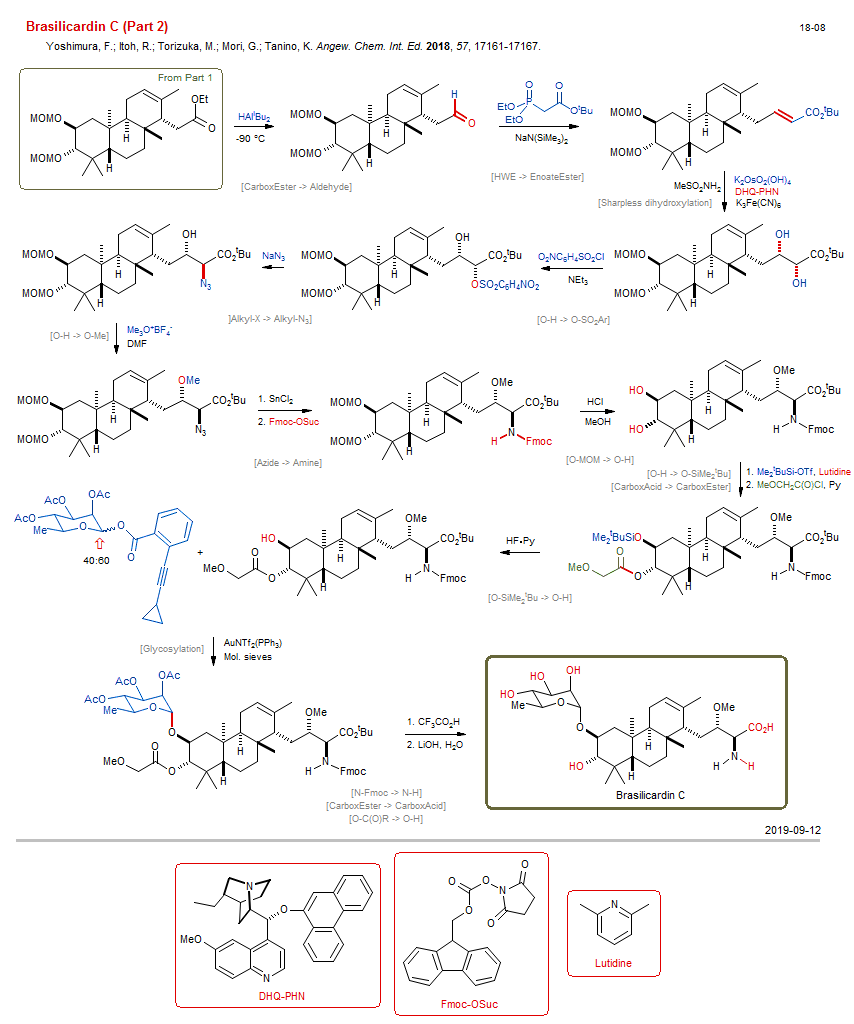
Total synthesis of Brasilicardin C (Part 2) New:
| Reference: | Yoshimura, F.; Itoh, R.; Torizuka, M.; Mori, G.; Tanino, K. Angew. Chem. Int. Ed. 2018, 57, 17161-17167. DOI (Part 1) |
| Reactions: | CarboxEster → Aldehyde • CompRed-CarboxEster/Alkene+M-H • HWE → EnoateEster • Sharpless dihydroxylation • Alkene → Diol-1,2 (asym) • CompOx-Enoate/Alkene+OsO4 • O-H → O-SO2Ar • CompNu-O-H/O-H+RSO2X • O-H → O-Me • Azide → Amine • O-MOM → O-H • CompNu-O-MOM/CO2tBu+H+ • O-H → O-SiMe2tBu • CarboxAcid → CarboxEster • O-SiMe2tBu → O-H • Glycosylation • N-Fmoc → N-H • CarboxEster → CarboxAcid • O-C(O)R → O-H • |
| Reagents: | AlHiBu2 • Fe(CN)6K3 • Sulfonamide, methane • NaN(SiMe3)2 • NEt3 • Azide, sodium • Me3O+ X- • DMF (solvent) • SnCl2 • HCl • SiMe2tBu-OTf • Pyridine • HF·Py • AuNTf2 • Acetic acid, trifluoro • Hydroxide, lithium • Lutidine • |