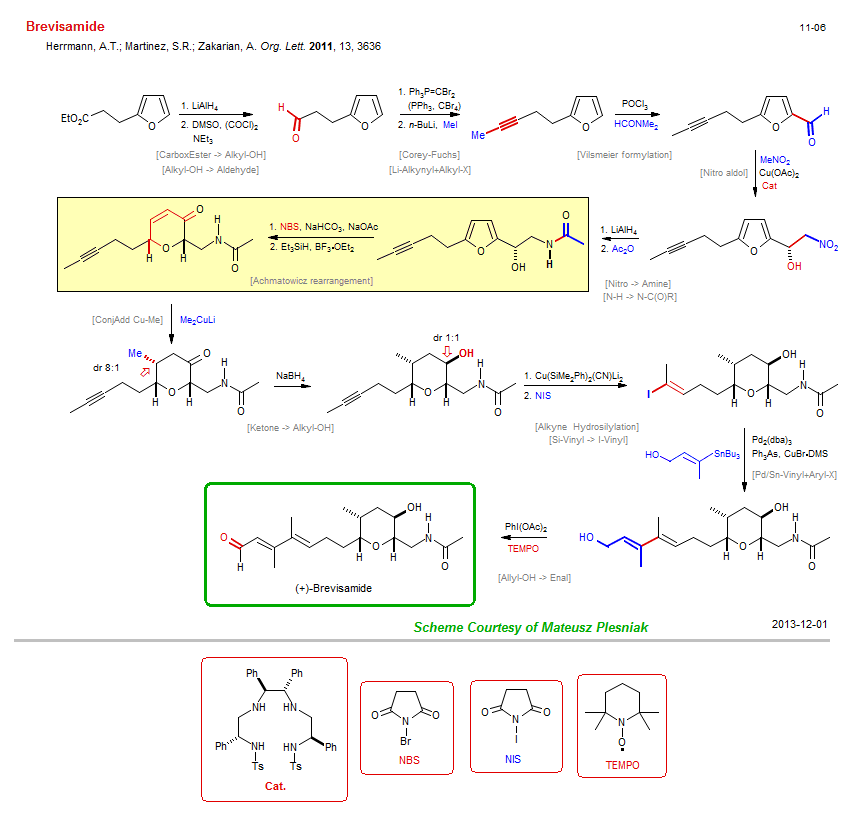
Total synthesis of Brevisamide:
| Reference: | Herrmann, A.T.; Martinez, S.R.; Zakarian, A. Org. Lett. 2011, 13, 3636 DOI |
| Reactions: | Corey-Fuchs • Vilsmeier formylation • Nitro aldol • Achmatowicz rearrangement • Ether-6 • Cyclohexenone • Pd/Sn-Vinyl+Aryl-X • Reductive etherification • ConjAdd Cu-Me • Cu-Me+Enone • Enone+Cu-Me • Alkyne Hydrosilylation • Cu-SiR3+Alkyne • Allyl-OH → Enal • CompOx-Allyl-OH/Alkyl-OH+Iodoso • Li-Alkynyl+Alkyl-X • CarboxEster → Alkyl-OH • Alkyl-OH → Aldehyde • Nitro → Amine • N-H → N-C(O)R • Ketone → Alkyl-OH • Si-Vinyl → I-Vinyl • |
| Reagents: | AlH4-Li+ • PPh3=CBr2 • POCl3 • Cu(OAc)2 • AlH4-Li+ • NBS • Me-Cuprate • BH4-Na+ • SiR3-Li • AsPh3 • TEMPO • DMSO, (COCl)2 (Swern) • BuLi(n) • Acetic anhydride • NIS • BF3·OEt2 • Vinyl-SnR3 • DMF (react) • MeNO2 • Iodosobenzene-OAc • Chiral catalyst (diamine) • MeI • PPh3, CBr4 • |