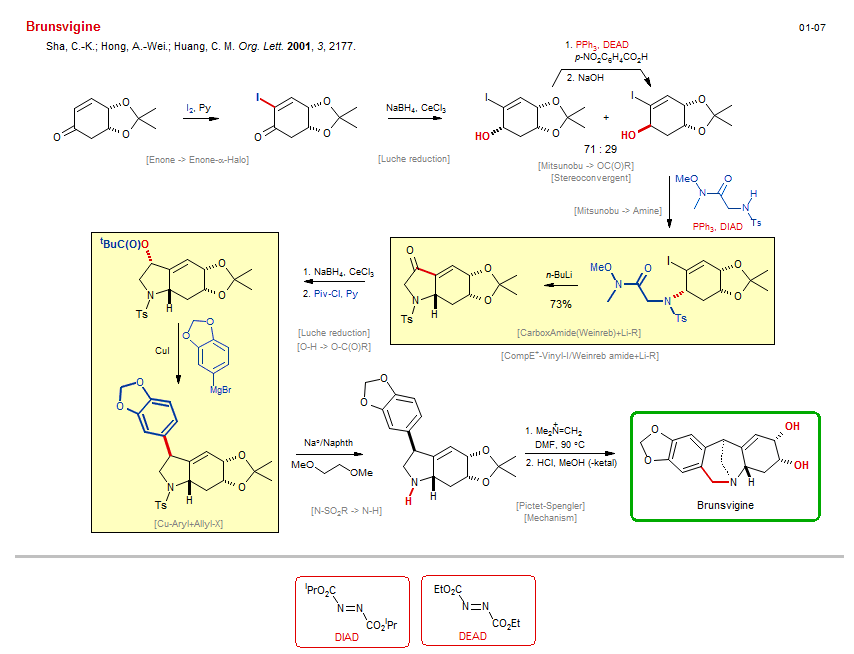
Total synthesis of Brunsvigine:
| Reference: | Sha, C.-K.; Hong, A.-Wei.; Huang, C. M. Org. Lett. 2001, 3, 2177. DOI |
| Reactions: | Ketal → Diol-1,2 • Pictet-Spengler • Amine-6 • Bicyclo(321)-aza • Mechanism • Cu-Aryl+Allyl-X • Allyl-X+Cu-R • Mg-R → Cu-R • Enone → Enone-α-Halo • Mitsunobu → OC(O)R • Alkyl-OH → Alkyl-OC(O)R • Alkyl-OH inversion • Stereoconvergent • CarboxAmide(Weinreb)+Li-R • Li-Vinyl+CarboxAmide • Li/I → Li-Vinyl • Amine-5 • Bicyclo(430)-aza • CompE+-Vinyl-I/Weinreb amide+Li-R • Mitsunobu → Amine • Allyl-OH → Allyl-NR2(2°) • Luche reduction • Enone → Allyl-OH • Luche reduction • Enone → Allyl-OH • O-H → O-C(O)R • N-SO2R → N-H • |
| Reagents: | I2 • BH4-Na+, CeCl3 • Benzoic acid, p-nitro • Hydroxide, sodium • Weinreb acetamide • PPh3, DIAD • BH4-Na+, CeCl3 • Piv-Cl • Aryl-MgX • CuI • Na°/Naphth • CH2=NMe2+I- • DMF (solvent) • HCl, MeOH • PPh3, DEAD • Vinyl-Li • BuLi(n) • |