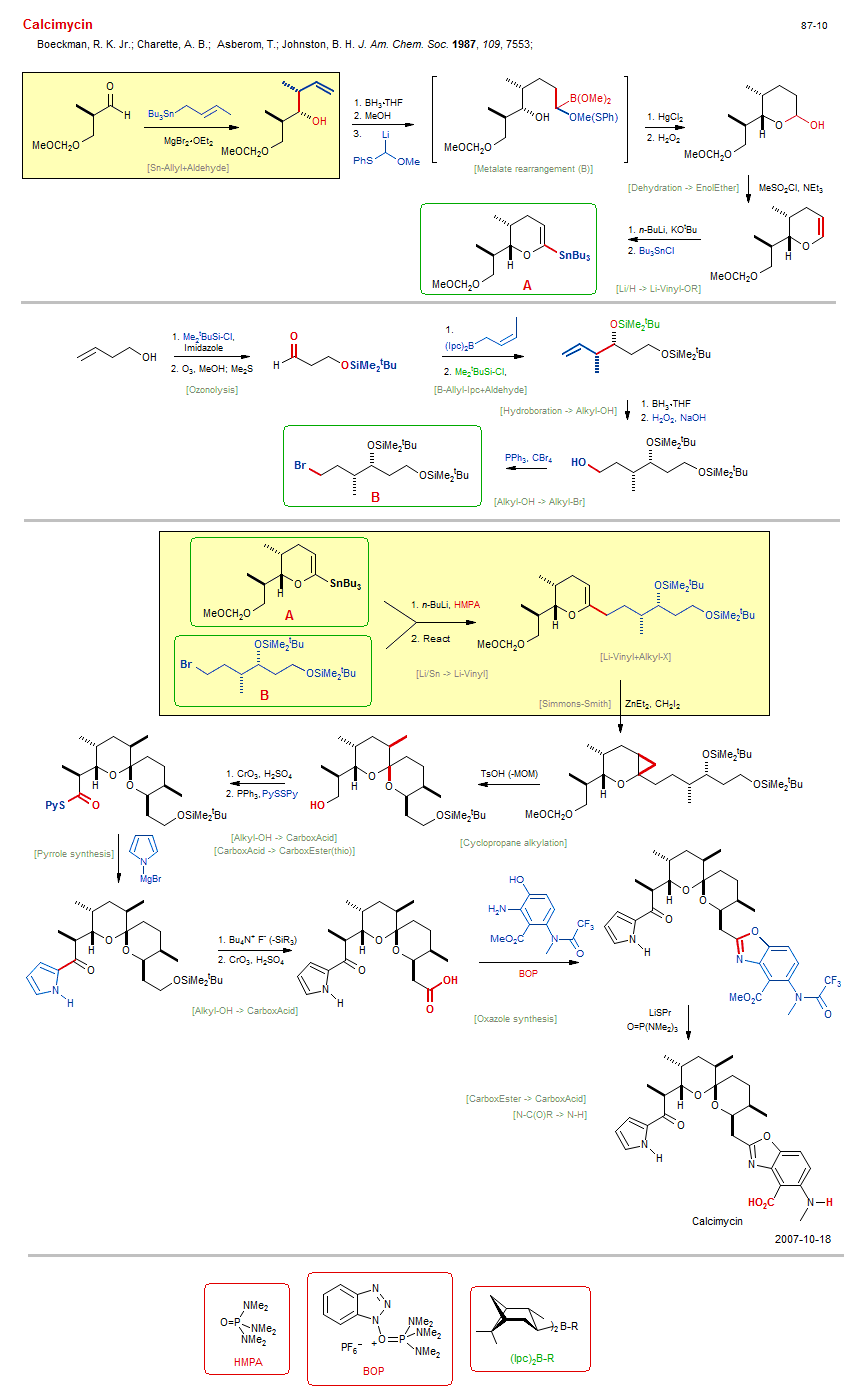
Total synthesis of Calcimycin:
| Reference: | Boeckman, R. K. Jr.; Charette, A. B.; Asberom, T.; Johnston, B. H. J. Am. Chem. Soc. 1987, 109, 7553; DOI |
| Reactions: | Li-Sulfide+Borane • Metalate rearrangement (B) • B-Alkyl → HO-Alkyl • Dehydration → EnolEther • Ether-6 • Sn-Allyl+Aldehyde • Aldehyde+Sn-R • Felkin-Anh Chelation • Li-R → Sn-R • Li/H → Li-Vinyl-OR • Ozonolysis • O-H → O-SiMe2tBu • B-Allyl-Ipc+Aldehyde • Aldehyde+B-Allyl (asym) • Hydroboration → Alkyl-OH • Alkyl-OH → Alkyl-Br • Appel → Br • Simmons-Smith • Cyclopropane • Li/Sn → Li-Vinyl • Li-Vinyl+Alkyl-X • Alkyl-X+Li-R • O-MOM → O-H • Jones oxidation • Cyclopropane alkylation • Spiroketal • Ether-6 • Alkyl-OH → CarboxAcid • Pyrrole synthesis • Mg-Aryl+CarboxEster • CarboxAcid → CarboxEster(thio) • O-SiMe2tBu → O-H • Jones oxidation • Oxazole synthesis • Alkyl-OH → CarboxAcid • CarboxEster → CarboxAcid • N-C(O)R → N-H • |
| Reagents: | Crotyl-SnR3 • MgBr2 • BH3·THF • LiCHR-SPh • HOMe (react) • HgCl2 • H2O2 • Sulfonyl chloride, methane, NEt3 • BuLi, KOtBu • SnBu3-Cl • Vinyl-SnR3 • SiMe2tBu-Cl • O3 • Me2S • Imidazole • Crotyl-B(Ipc)2 • Chiral auxiliary (boronate) • SiMe2tBu-Cl • BH3·THF • H2O2, NaOH • PPh3, CBr4 • CH2I2, ZnEt2 • BuLi(n), HMPA • Vinyl-SnR3 • TsOH • CrO3, H2SO4 (Jones) • PPh3 • Disulfide, pyridyl • Pyrrolyl-MgX • Fluoride, R4N+ • CrO3, H2SO4 (Jones) • BOP • Thiolate, propyl • HMPA (solvent) • |