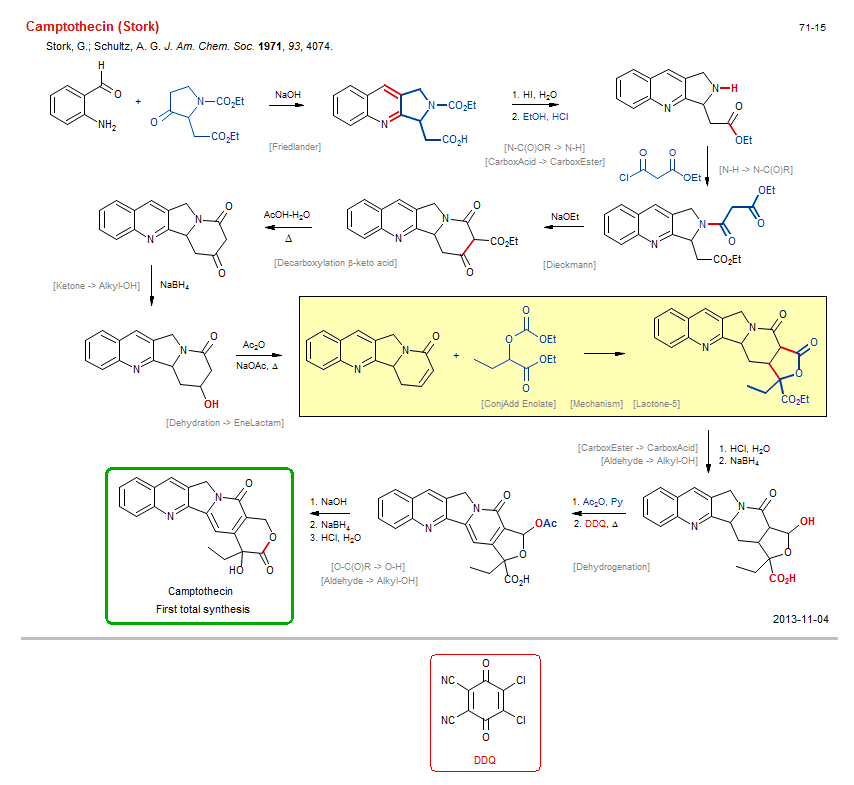
Total synthesis of Camptothecin (Stork):
| Reference: | Stork, G.; Schultz, A. G. J. Am. Chem. Soc. 1971, 93, 4074. DOI |
| Reactions: | O-H → O-C(O)R • Friedlander • Quinoline synthesis • Dieckmann • CarboxEster enolate+CarboxEster • Lactam-6 • Decarboxylation β-keto acid • Dehydration → EneLactam • ConjAdd Enolate • Dehydrogenation • Mechanism • N-C(O)OR → N-H • N-H → N-C(O)R • Ketone → Alkyl-OH • CompRed-Ketone/CarboxAmide+M-H • Lactone-5 • CarboxEster → CarboxAcid • Aldehyde → Alkyl-OH • CompRed-CarboxEster/CarboxAmide+M-H • Aldehyde → Alkyl-OH • Lactone-6 • O-C(O)R → O-H • CarboxAcid → CarboxEster • Fischer esterification • |
| Reagents: | Hydroxide, sodium • HI • HCl, EtOH • Malonate • NaOEt • BH4-Na+ • Acetic acid • Acetic anhydride • Acetate, sodium • HCl, H2O • BH4-Na+ • Acetic anhydride, Py • DDQ • Hydroxide, sodium • BH4-Na+ • HCl, H2O • |