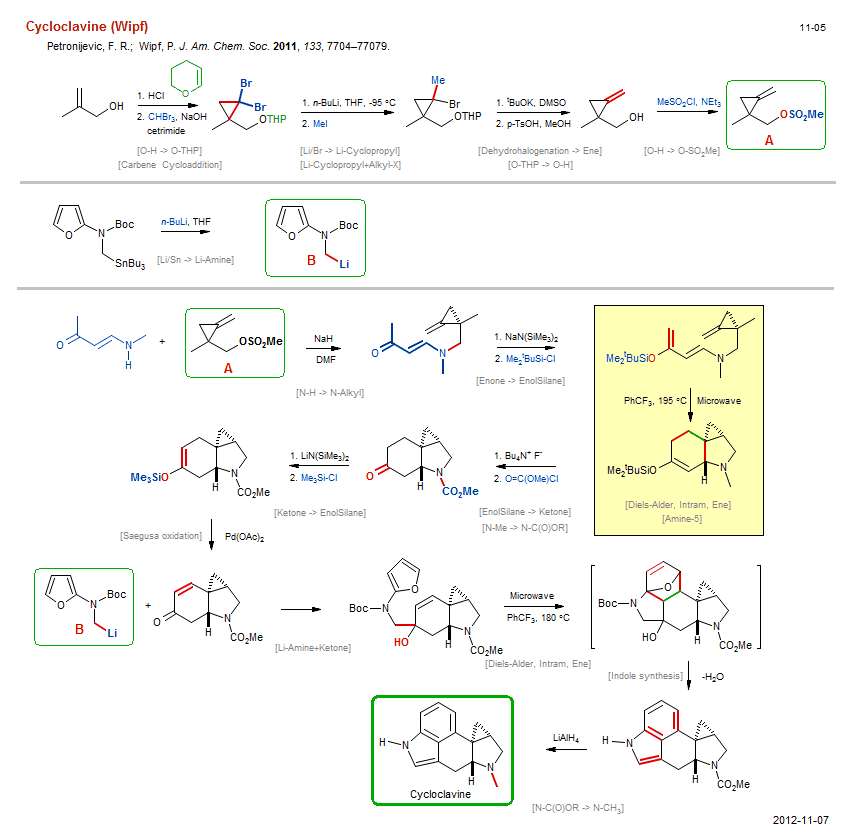
Total synthesis of Cycloclavine (Wipf):
| Reference: | Petronijevic, F. R.; Wipf, P. J. Am. Chem. Soc. 2011, 133, 7704–77079. DOI |
| Reactions: | Carbene Cycloaddition • Cyclopropane • Li/Br → Li-Cyclopropyl • Dehydrohalogenation → Ene • N-H → N-Alkyl • Enone → EnolSilane • Diels-Alder, Intram, Ene • Amine-5 • Bicyclo(430)-aza • Bicyclo(310)-aza • N-Me → N-C(O)OR • Ketone → EnolSilane • Saegusa oxidation • Li-Amine+Ketone • Ketone+Li-Alkyl • CompE+-Ketone/Carbamate+M-R • Diels-Alder, Intram, Ene • Furan cycloaddition • Indole synthesis • N-C(O)OR → N-CH3 • Li/Sn → Li-Amine • Amine-5 • O-H → O-SO2Me • EnolSilane → Ketone • O-H → O-THP • Li-Cyclopropyl+Alkyl-X • Alkyl-X+Li-R • O-THP → O-H • |
| Reagents: | HCl • CHBr3 • Imidazole • BuLi(n) • MeI • KOtBu • TsOH • Sulfonyl chloride, methane, NEt3 • Butadiene, 2-OR • Butadiene, 1-NR2 • NaN(SiMe3)2 • SiMe2tBu-Cl • Microwave • O=C(OMe)Cl • Fluoride, R4N+ • LiN(SiMe3)2 • SiMe3-Cl • Pd(OAc)2 • BuLi(n) • Microwave • AlH4-Li+ • NaH • DMF (solvent) • DHP • |