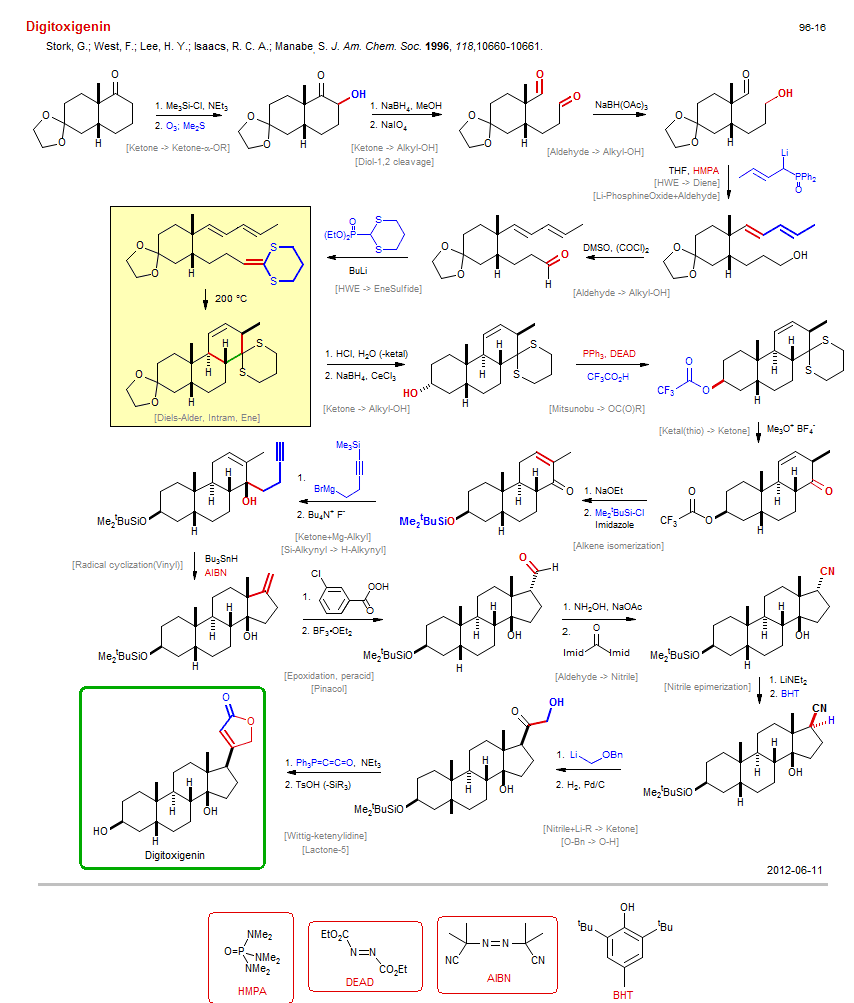
Total synthesis of Digitoxigenin:
| Reference: | Stork, G.; West, F.; Lee, H. Y.; Isaacs, R. C. A.; Manabe, S. J. Am. Chem. Soc. 1996, 118,10660-10661. DOI |
| Reactions: | Diol-1,2 cleavage • Li-PhosphineOxide+Aldehyde • Aldehyde+Li-Allyl • Ketal → Ketone • O-H → O-SiMe2tBu • Aldehyde → Oxime • O-SiMe2tBu → O-H • Ketone → Ketone-α-OR • Diol-1,2 cleavage • Aldehyde → Alkyl-OH • CompRed-Aldehyde/Aldehyde+M-H • HWE → Diene • Aldehyde → Alkyl-OH • HWE → EneSulfide • Diels-Alder, Intram, Ene • Diels-Alder, EneSulfide • Cyclohexane • Bicyclo(440) • Mitsunobu → OC(O)R • Alkyl-OH → Alkyl-OC(O)R • Alkyl-OH inversion • Ketal(thio) → Ketone • Ketone+Mg-Alkyl • Pinacol • Epoxide rearrangement • Aldehyde → Nitrile • Oxime → Nitrile • Nitrile epimerization • Nitrile+Li-R → Ketone • Li-Ether+Nitrile • Lactone-5 • Alkene isomerization • Radical cyclization(Vinyl) • Cyclopentane • Bicyclo(430) • Wittig-ketenylidine • Wittig (intram) • Si-Alkynyl → H-Alkynyl • CompE+-Si-C/Si-O+H2O • Epoxidation, peracid • O-Bn → O-H • Ketone → Alkyl-OH • Li-PhosphineOxide+Aldehyde • Ketone → Alkyl-OH • |
| Reagents: | SiMe3-Cl • O3; Me2S • BH4-Na+ • Periodate, sodium • BH(OAc)3-Na+ • P(O)Ph2CH2-R • Allyl-Li • HMPA (solvent) • DMSO, (COCl)2 (Swern) • P(O)(OR)2CH2-SR' • EneSulfide • BuLi(n) • HCl, H2O • BH4-Na+, CeCl3 • PPh3, DEAD • Acetic acid, trifluoro • Me3O+ X- • NaOEt • SiMe2tBu-Cl • Imidazole • Alkynyl-SiR3 • Alkyl-MgX • Fluoride, R4N+ • HSnBu3 • AIBN • BF3·OEt2 • NH2OH • O=C(Imid)2 • LiCH2-OR • LiNEt2 • BHT • H2, Pd • PPh3=C=C=O • NEt3 • TsOH • BHT • HMPA (solvent) • Perbenzoic acid, 3-chloro • |