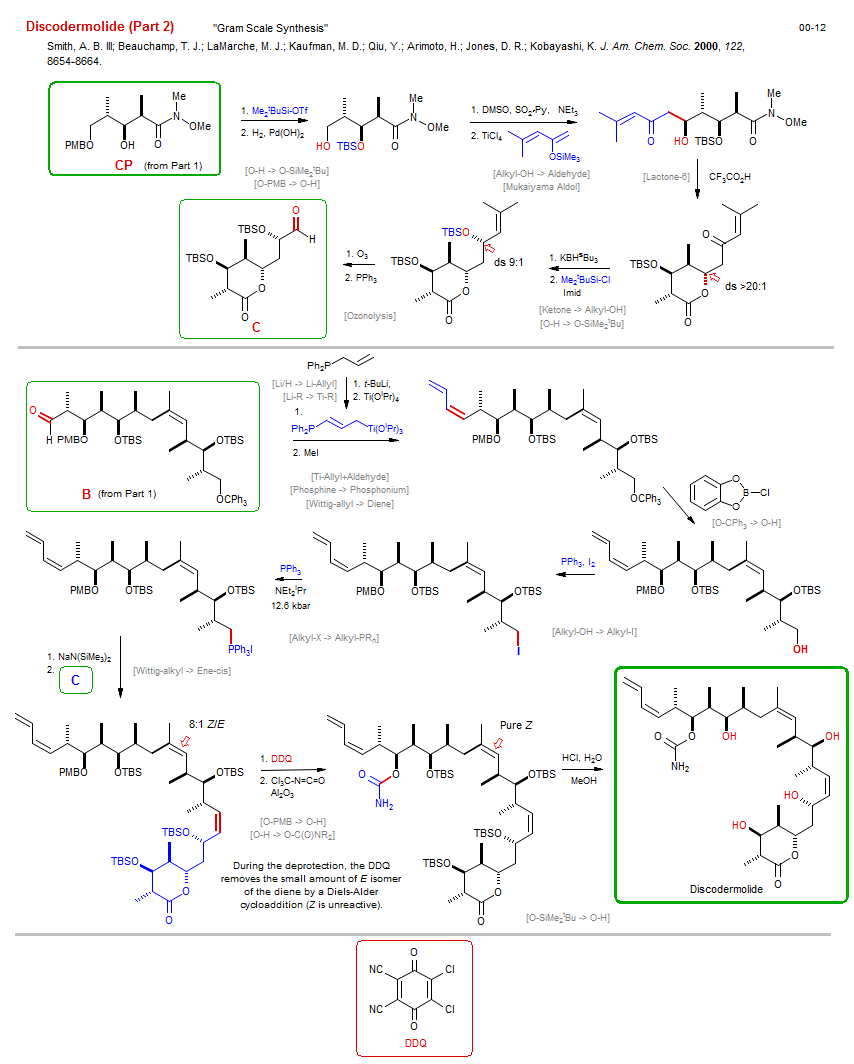
Total synthesis of Discodermolide (Part 2):
| Reference: | Smith, A. B. III; Beauchamp, T. J.; LaMarche, M. J.; Kaufman, M. D.; Qiu, Y.; Arimoto, H.; Jones, D. R.; Kobayashi, K. J. Am. Chem. Soc. 2000, 122, 8654-8664. DOI |
| Reactions: | Alkyl-X → Alkyl-PRn • Wittig-alkyl → Ene-cis • O-H → O-SiMe2tBu • O-PMB → O-H • Alkyl-OH → Aldehyde • Mukaiyama Aldol • Aldol-Si • Aldehyde+EnolSilane • Lactone-6 • Ketone → Alkyl-OH • O-H → O-SiMe2tBu • Ozonolysis • O-PMB → O-H • O-H → O-C(O)NR2 • O-SiMe2tBu → O-H • Ti-Allyl+Aldehyde • Ti-Phosphine+Aldehyde • Wittig-allyl → Diene • Alkyl-OH → Alkyl-I • O-CPh3 → O-H • Li/H → Li-Allyl • Li/H → Li-Phosphine • Li-R → Ti-R • Phosphine → Phosphonium • |
| Reagents: | SiMe2tBu-OTf • H2, Pd • Pyridine • NEt3 • TiCl4 • Butadiene, 2-OR • Acetic acid, trifluoro • BH(sBu3)-K+ • SiMe2tBu-Cl • O3 • PPh3 • PPh3 • High pressure • NaN(SiMe3)2 • DDQ • Isocyanate • Al2O3 • HCl, H2O • Allyl-Ti • MeI • B(Cl)Catechol • I2 • BuLi(tert) • Ti(OiPr)4 • |