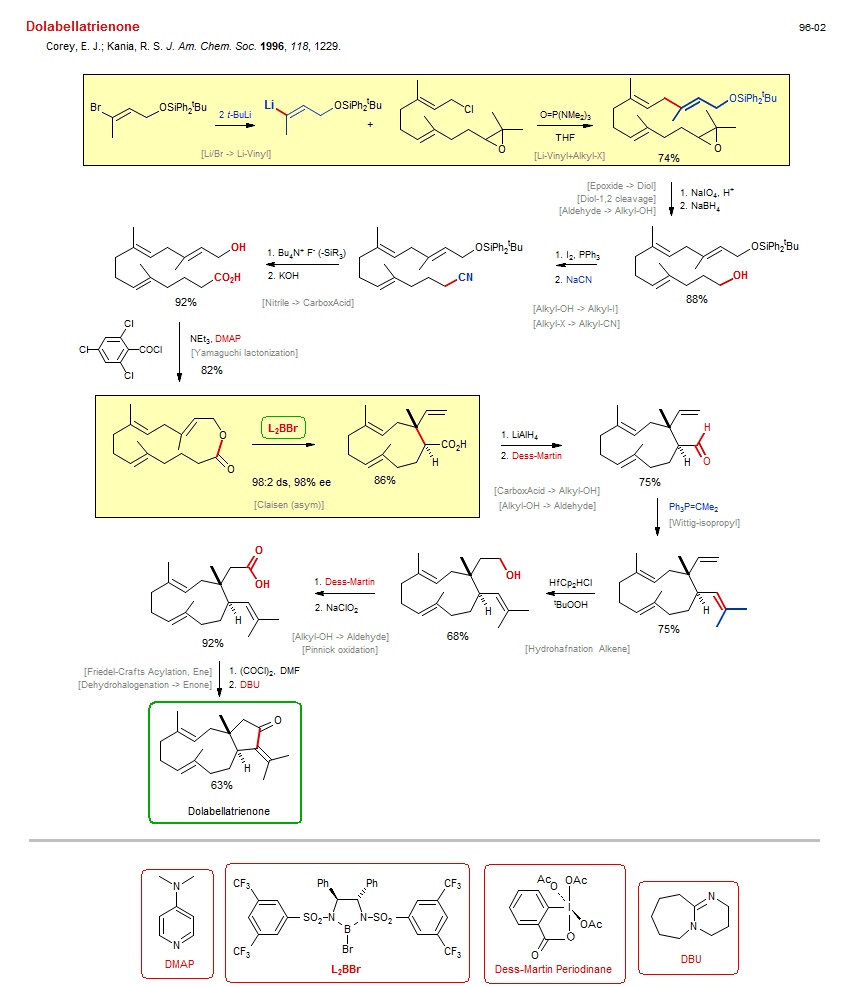
Total synthesis of Dolabellatrienone:
| Reference: | Corey, E. J.; Kania, R. S. J. Am. Chem. Soc. 1996, 118, 1229. DOI |
| Reactions: | O-SiPh2tBu → O-H • CarboxAcid → CarboxCl • Li/Br → Li-Vinyl • Yamaguchi lactonization • Lactone-9+ • Wittig-isopropyl • Friedel-Crafts Acylation, Ene • Li-Vinyl+Alkyl-X • Allyl-X+Li-R • Hydrohafnation Alkene • Nitrile → CarboxAcid • Claisen (asym) • Cycloundecane • Epoxide → Diol • Diol-1,2 cleavage • Aldehyde → Alkyl-OH • Alkyl-OH → Alkyl-I • Alkyl-X → Alkyl-CN • CarboxAcid → Alkyl-OH • Alkyl-OH → Aldehyde • Alkyl-OH → Aldehyde • Pinnick oxidation • Aldehyde → CarboxAcid • Dehydrohalogenation → Enone • Diol-1,2 cleavage • |
| Reagents: | BuLi(tert) • Vinyl-Li • Epoxide • HMPA (solvent) • Periodate, sodium • BH4-Na+ • PPh3, I2 • Cyanide, sodium • Fluoride, R4N+ • Hydroxide, potassium • Benzoyl chloride, 2,4,6-trichloro • NEt3 • Corey diazaborolidine • AlH4-Li+ • Dess-Martin • PPh3=CMe2 • HfCp2HCl • Hydroperoxide, tbutyl • Dess-Martin • Chlorite, sodium • Oxalyl chloride • DBU • DMAP • DMF (solvent) • Chiral catalyst (diamine) • |