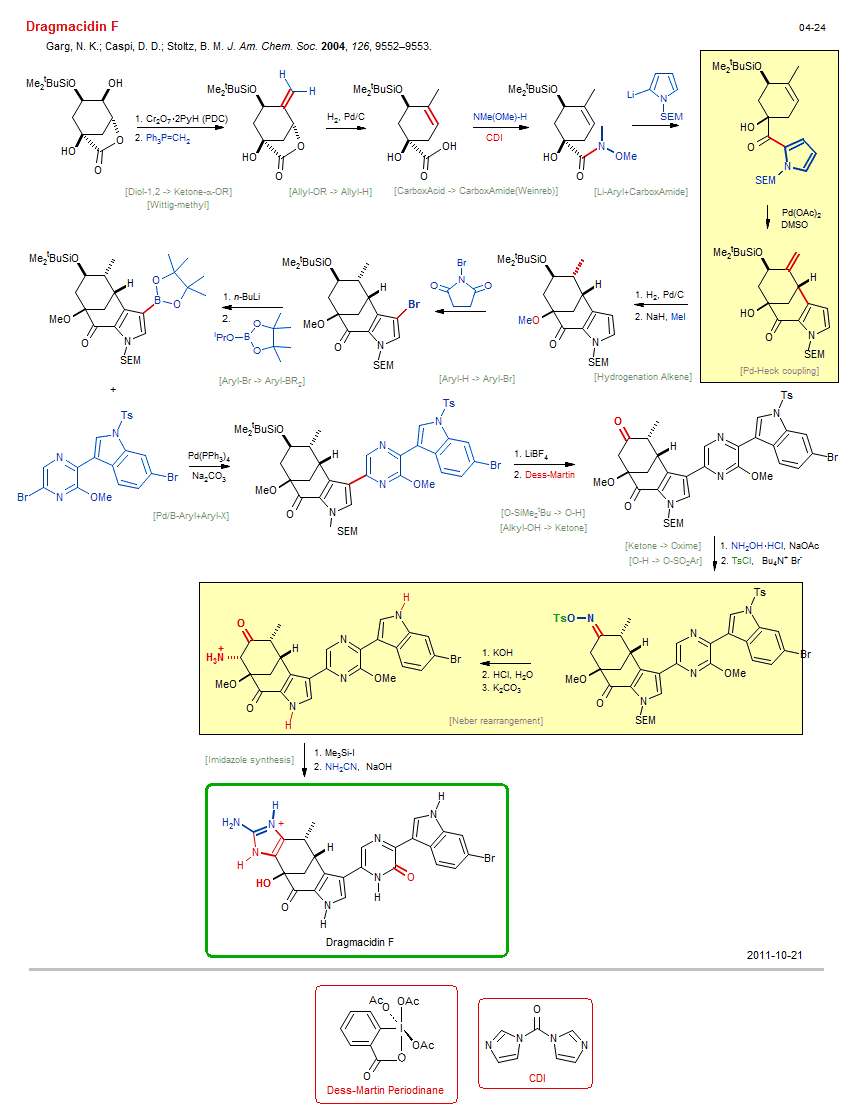
Total synthesis of Dragmacidin F:
| Reference: | Garg, N. K.; Caspi, D. D.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 9552–9553. DOI |
| Reactions: | Li/Br → Li-Aryl • Neber rearrangement • Ketone → Ketone-α-NR2 • O-Me → O-H • Pd-Heck coupling • Cyclohexanone • Bicyclo(331) • Wittig-methyl • Allyl-OR → Allyl-H • CarboxAcid → CarboxAmide(Weinreb) • Li-Aryl+CarboxAmide • CarboxAmide(Weinreb)+Li-R • Hydrogenation Alkene • Aryl-H → Aryl-Br • Aryl-Br → Aryl-BR2 • CompE+-Aryl-Br/Ketone+M-R • Pd/B-Aryl+Aryl-X • Imidazole synthesis • O-H → O-Me • Williamson etherification • Diol-1,2 → Ketone-α-OR • Alkyl-OH → Ketone • O-SiMe2tBu → O-H • O-H → O-SO2Ar • Ketone → Oxime • |
| Reagents: | Cr2O7·2PyH (PDC) • PPh3=CH2 • H2, Pd • NHMe(OMe) • Carbodiimide • Pyrrolyl-Li • Pd(OAc)2 • DMSO (solvent) • H2, Pd • NaH • Pyrrolyl-Li • BuLi(n) • B(OR)3 • Pd(PPh3)4 • Carbonate, sodium • LiBF4 • Dess-Martin • NH2OH • TsCl • Bromide, R4N+ • Hydroxide, potassium • HCl, H2O • Carbonate, potassium • SiMe3-I • MeI • Hydroxide, sodium • NBS • |