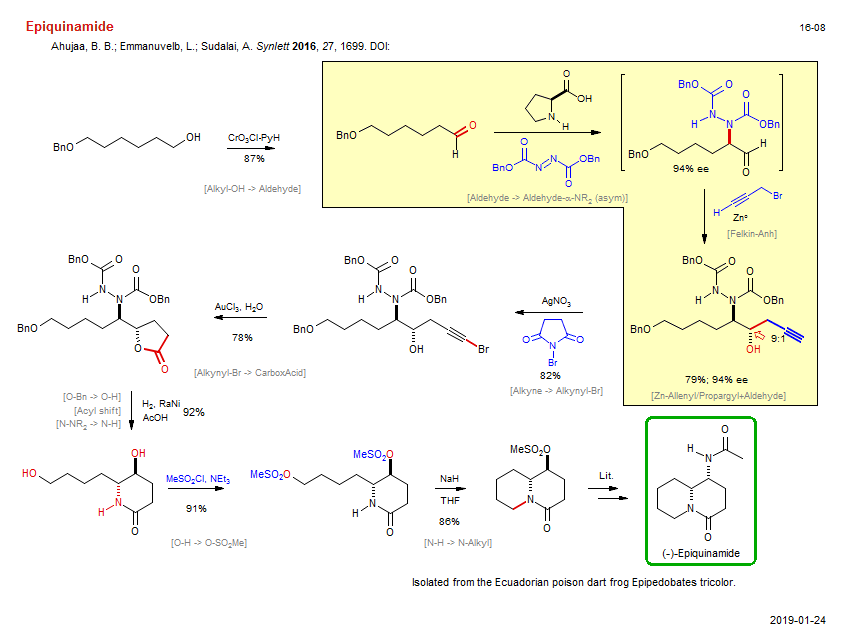
Total synthesis of Epiquinamide:
| Reference: | Ahujaa, B. B.; Emmanuvelb, L.; Sudalai, A. Synlett 2016, 27, 1699. DOI: DOI |
| Reactions: | Alkyl-OH → Aldehyde • Alkyne → Alkynyl-Br • Alkynyl-Br → CarboxAcid • Lactone-5 • O-Bn → O-H • Acyl shift • Lactam-6 • O-H → O-SO2Me • N-H → N-Alkyl • Amine-6 • Bicyclo(440)-aza • Aldehyde → Aldehyde-α-NR2 (asym) • Enamine+Azo • Felkin-Anh • Zn-Allenyl/Propargyl+Aldehyde • N-NR2 → N-H • |
| Reagents: | CrO3Cl·PyH (PCC) • AgNO3 • NBS • AuCl3 • H2, RaNi • Acetic acid • Sulfonyl chloride, methane, NEt3 • NaH • Proline • DEAD • Propargyl-Br • Zn° • |