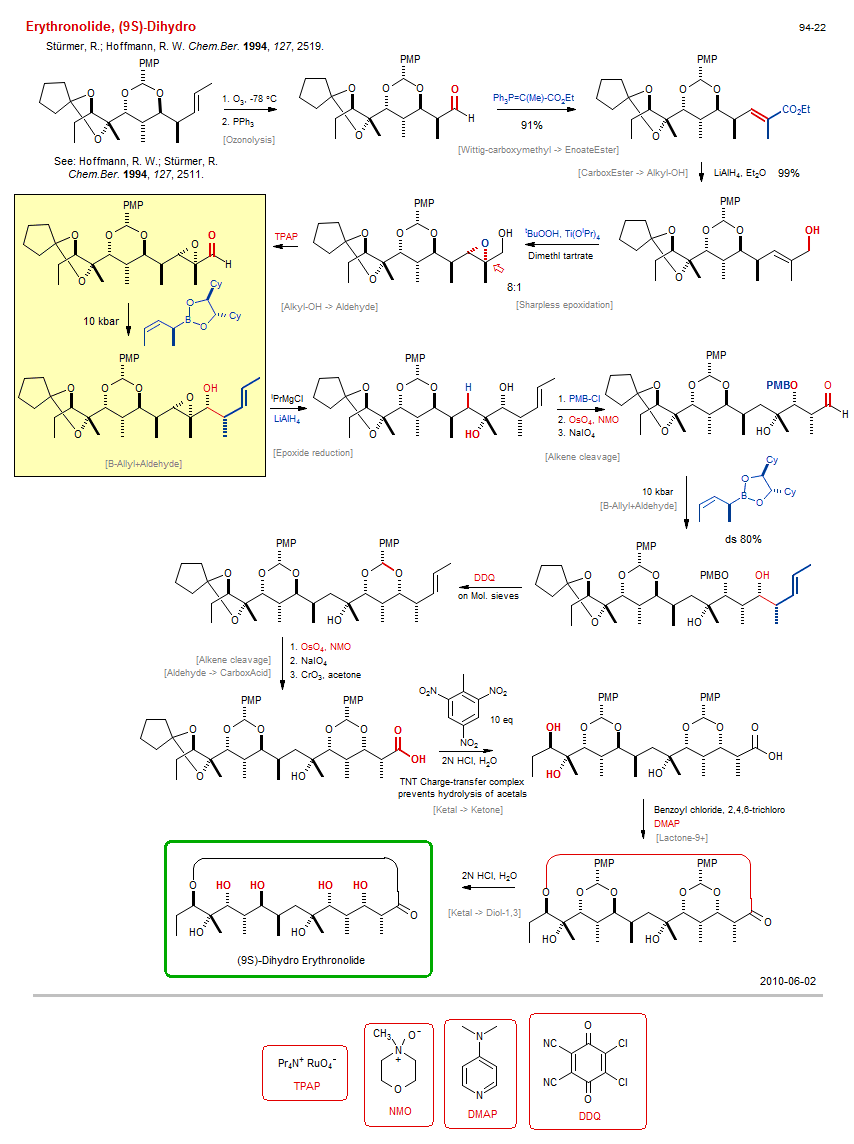
Total synthesis of Erythronolide, (9S)-Dihydro :
| Reference: | Stürmer, R.; Hoffmann, R. W. Chem.Ber. 1994, 127, 2519. DOI |
| Reactions: | O-H → O-PMB • Ozonolysis • Wittig-carboxymethyl → EnoateEster • Sharpless epoxidation • B-Allyl+Aldehyde • Aldehyde+B-Allyl (asym) • High pressure • Epoxide reduction • Alkene cleavage • Alkene cleavage • Lactone-9+ • Ketal → Diol-1,3 • B-Allyl+Aldehyde • High pressure • CarboxEster → Alkyl-OH • Alkyl-OH → Aldehyde • Aldehyde → CarboxAcid • Ketal → Ketone • |
| Reagents: | O3 • PPh3 • PPh3=C(Me)-CO2R • AlH4-Li+ • Hydroperoxide, tbutyl - Ti • RuO4-Pr4N+ • Alkyl-MgX • AlH4-Li+ • PMB-Cl • OsO4, NMO • Periodate, sodium • Crotyl-B(Cy2-diol) • Chiral auxiliary (boronate) • High pressure • DDQ • HCl, H2O • OsO4, NMO • Periodate, sodium • CrO3 • HCl, H2O • Benzoyl chloride, 2,4,6-trichloro • DMAP • Crotyl-B(Cy2-diol) • Chiral auxiliary (boronate) • |