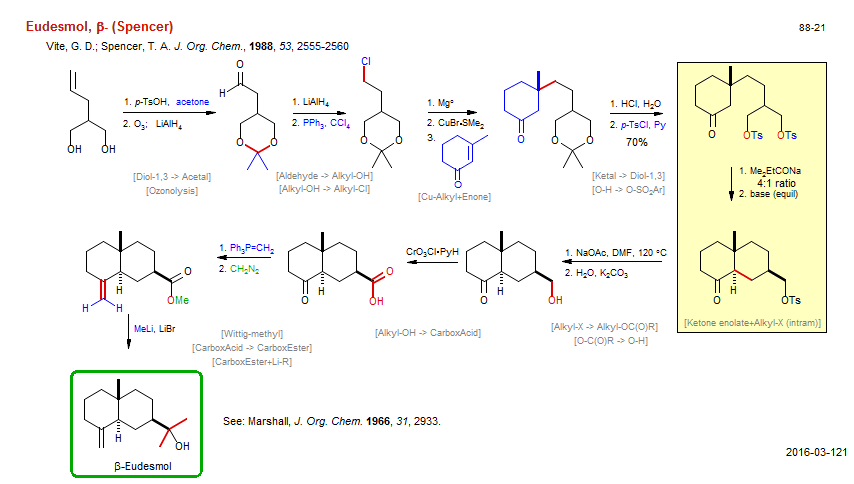
Total synthesis of Eudesmol, β- (Spencer):
| Reference: | Vite, G. D.; Spencer, T. A. J. Org. Chem., 1988, 53, 2555-2560DOI |
| Reactions: | Diol-1,3 → Acetal • Ozonolysis • Cu-Alkyl+Enone • Enone+Cu-R • Mg-R → Cu-R • Ketone enolate+Alkyl-X (intram) • Alkyl-X+Enolate (intram) • Cyclohexane • Bicyclo(440) • Alkyl-X → Alkyl-OC(O)R • CarboxEster+Li-R • Li-Me+CarboxEster • O-H → O-SO2Ar • Alkyl-OH → CarboxAcid • Wittig-methyl • Aldehyde → Alkyl-OH • Alkyl-OH → Alkyl-Cl • Ketal → Diol-1,3 • CarboxAcid → CarboxEster • O-C(O)R → O-H • |
| Reagents: | TsOH • O3 • AlH4-Li+ • PPh3, CCl4 • Alkyl-MgX • CuBr·SMe2 • HCl, H2O • TsCl, Py • NaOtAm • Acetate, sodium • Carbonate, potassium • CrO3Cl·PyH (PCC) • Diazomethane • Me-Li • AlH4-Li+ • PPh3=CH2 • |