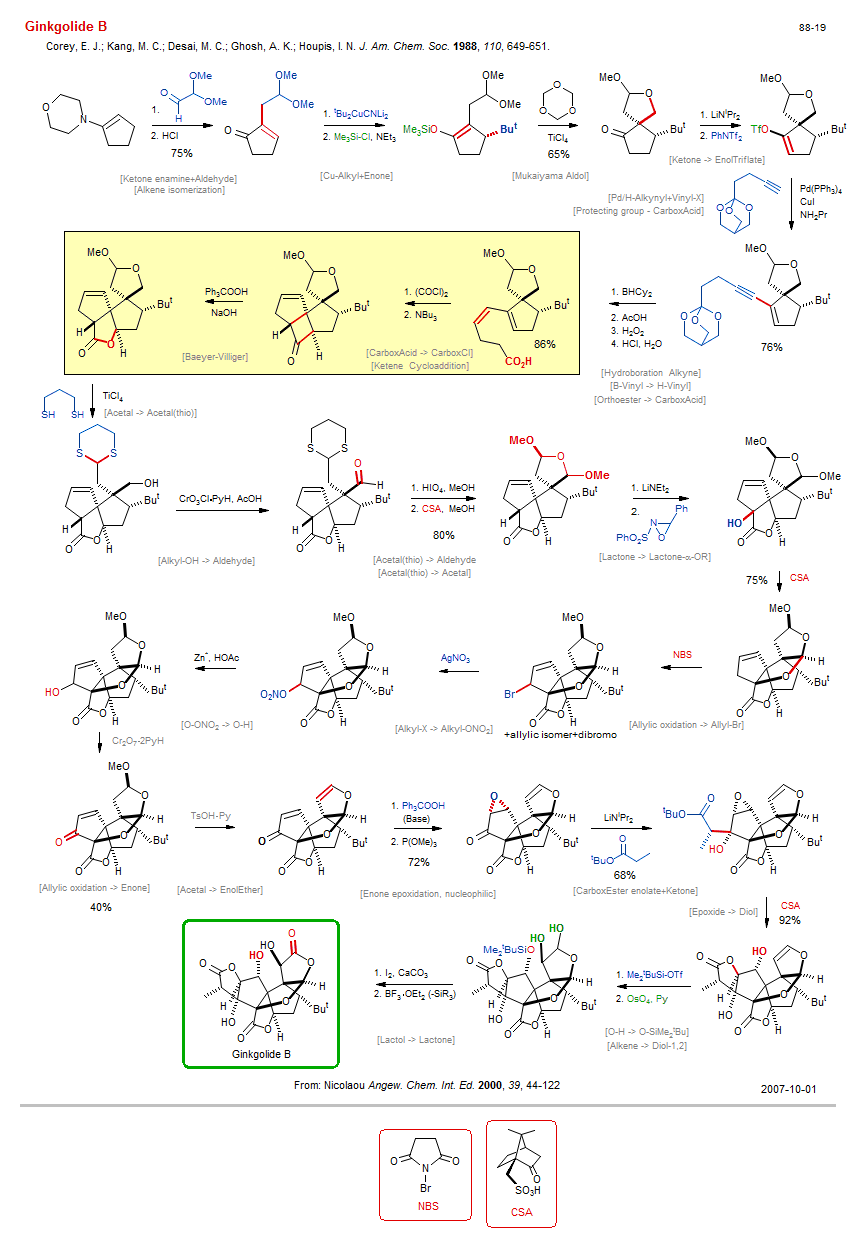
Total synthesis of Ginkgolide B:
| Reference: | Corey, E. J.; Kang, M. C.; Desai, M. C.; Ghosh, A. K.; Houpis, I. N. J. Am. Chem. Soc. 1988, 110, 649-651. DOI |
| Reactions: | Pd/H-Alkynyl+Vinyl-X • Ketone enamine+Aldehyde • Aldol-enamine → Enone • Alkyl-OH → Aldehyde • CompOx-Alkyl-OH/Sulfide+CrO3 • Acetal → Acetal • Wohl-Ziegler bromination • Allylic oxidation → Enone • O-SiMe2tBu → O-H • Cu-Alkyl+Enone • Enone+Cu-R • ConjAdd-Silylation • ConjAdd Cu-Alkyl • Mukaiyama Aldol • Aldol-Si • Aldehyde+EnolSilane • Lactone → Lactone-α-OR • Oxaziridine+Enolate • CarboxEster enolate+Ketone • CompE+-Ketone/Lactone+Enolate • Epoxide → Diol • Lactone-5 • Lactol → Lactone • Lactone-5 • CompOx-Lactol/Alkyl-OH+I2 • Hydroboration Alkyne • CompRed-Alkyne/Alkene+M-H • Enone epoxidation, nucleophilic • CompOx-Enone/Alkene+ROOH • Protecting group - CarboxAcid • Baeyer-Villiger • Lactone-5 • Ketene Cycloaddition • Cyclobutane • Cyclopentene • CarboxCl → Ketene • Ketene synthesis • Bicyclo(320) • Acetal(thio) → Acetal • Alkene isomerization • Ketone → EnolTriflate • B-Vinyl → H-Vinyl • Alkyne → Ene-cis (B-H) • Orthoester → CarboxAcid • CarboxAcid → CarboxCl • Spiro(44) • Bicyclo(320) • Acetal → Acetal(thio) • Alkyl-X → Alkyl-ONO2 • O-ONO2 → O-H • Allylic oxidation → Allyl-Br • Wohl-Ziegler bromination • Alkene → Diol-1,2 • O-H → O-SiMe2tBu • CompNu-O-H/O-H+R3Si-X • Acetal → EnolEther • |
| Reagents: | HCl • CuCN • SiMe3-Cl • TiCl4 • Formaldehyde • LiNiPr2 • Tf2NPh • Pd(PPh3)4 • CuI • NH2Pr • BH(Cy)2 • Acetic acid • H2O2 • HCl, H2O • Thiol, (CH2)3-SH • TiCl4 • CrO3Cl·PyH (PCC) • Periodic acid • Sulfonic acid, Camphor • LiNEt2 • Oxaziridine • Sulfonic acid, Camphor • NBS • AgNO3 • TsOH·Py • Hydroperoxide, trityl • P(OMe)3 • LiNiPr2 • CH2=C(OLi)(OR) • Sulfonic acid, Camphor • SiMe2tBu-OTf • OsO4 • I2 • Carbonate, calcium • BF3·OEt2 • Oxalyl chloride • NBu3 • Hydroperoxide, trityl • Hydroxide, sodium • HOMe (react) • Acetic acid • Cr2O7·2PyH (PDC) • HOMe (react) • |