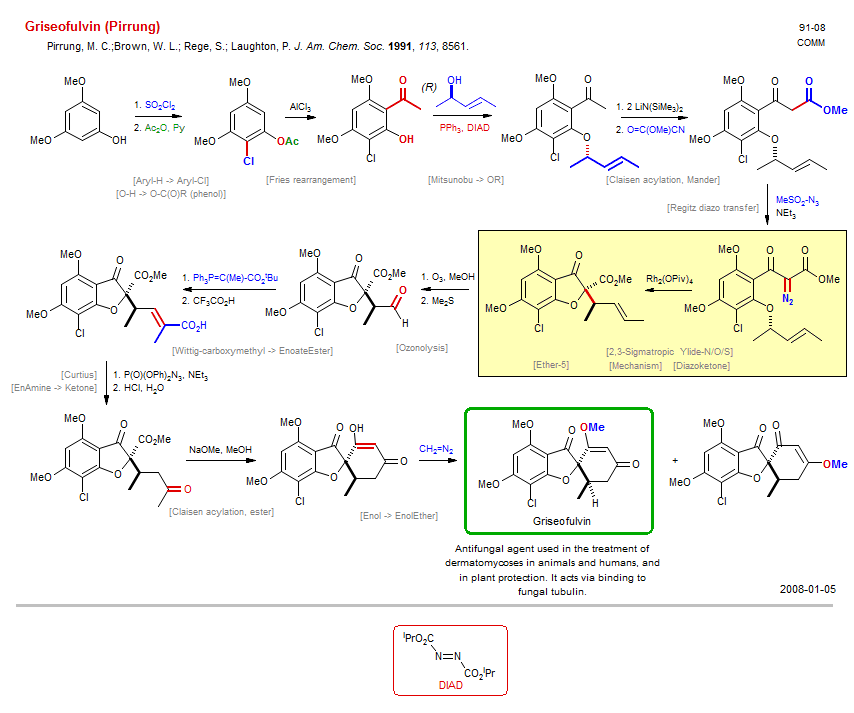
Total synthesis of Griseofulvin (Pirrung):
| Reference: | Pirrung, M. C.;Brown, W. L.; Rege, S.; Laughton, P. J. Am. Chem. Soc. 1991, 113, 8561. DOI |
| Reactions: | CarboxEster → CarboxAcid (tBu) • CarboxAcid → CarboxAzide • Fries rearrangement • Claisen acylation, Mander • Ketone enolate+Mander • Mander+enolate • Aryl-H → Aryl-Cl • Mitsunobu → OR • O-H → O-Alkyl (phenol) • Ozonolysis • Wittig-carboxymethyl → EnoateEster • CompE+-Aldehyde/Ketone+Wittig • Curtius • C-CO2H → C-N • Mechanism • Claisen acylation, ester • Ketone enolate+CarboxEster • Cyclohexenone • Spiro(54)-oxa • Ether-5 • 2,3-Sigmatropic Ylide-N/O/S • Oxonium ylide • Ether-5 • Diazoketone • Mechanism • Ether-5 • O-H → O-C(O)R (phenol) • Enol → EnolEther • Regitz diazo transfer • EnAmine → Ketone • |
| Reagents: | SO2Cl2 • Acetic anhydride, Py • AlCl3 • PPh3, DIAD • LiN(SiMe3)2 • O=C(OR)CN • NEt3 • O3 • Me2S • PPh3=C(Me)-CO2R • Acetic acid, trifluoro • Azide, diphenylphosphoryl • HCl, H2O • NaOMe • Diazomethane • Rh2(OPiv)4 • |