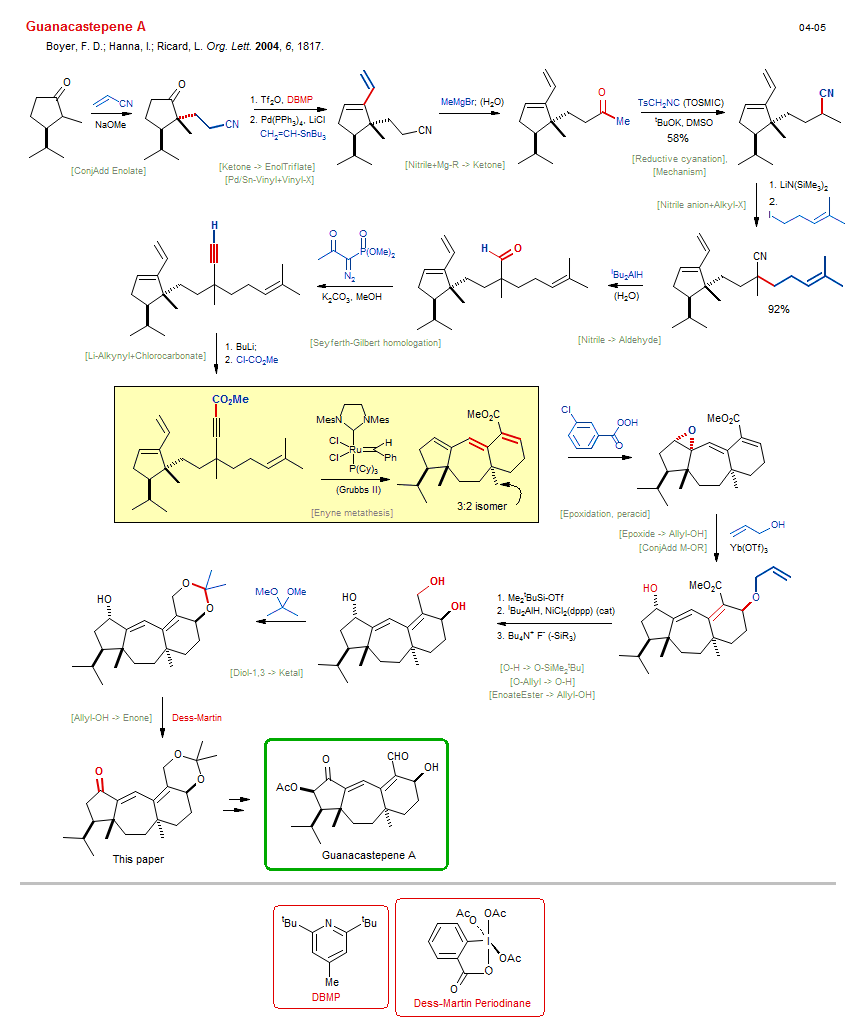
Total synthesis of Guanacastepene A:
| Reference: | Boyer, F. D.; Hanna, I.; Ricard, L. Org. Lett. 2004, 6, 1817. DOI |
| Reactions: | Pd/Sn-Vinyl+Vinyl-X • ConjAdd Enolate • Ketone enolate+EneNitrile • EneNitrile+Enolate • Li/H → Li-Alkynyl • O-SiMe2tBu → O-H • Seyferth-Gilbert homologation • Aldehyde → Alkyne • Nitrile anion+Alkyl-X • Li-Alkynyl+Chlorocarbonate • Enyne metathesis • Cyclohexene • Cycloheptane • Bicyclo(540) • Bicyclo(530) • Nitrile+Mg-R → Ketone • Mg-Alkyl+Nitrile • Nitrile → Aldehyde • Epoxide → Allyl-OH • Ketone → EnolTriflate • Epoxidation, peracid • CompOx-Alkene/Alkene+Peracid • Mechanism • Allyl-OH → Enone • Reductive cyanation • Li-Isocyanide • O-H → O-SiMe2tBu • EnoateEster → Allyl-OH • O-Allyl → O-H • ConjAdd M-OR • |
| Reagents: | Acrylonitrile • NaOMe • Pyridine, 2,6-di-tBu • Pd(PPh3)4 • Me-MgBr • TsCH2NC • LiN(SiMe3)2 • AlHiBu2 • Diazophosphonate • Carbonate, potassium • BuLi(n) • HO-Allyl • Yb(OTf)3 • SiMe2tBu-OTf • AlHiBu2 • Fluoride, R4N+ • Me2C(OMe)2 • Dess-Martin • Grubbs II • EneSulfide • NiCl2 • O=C(OMe)Cl • KOtBu • DMSO (solvent) • Perbenzoic acid, 3-chloro • |