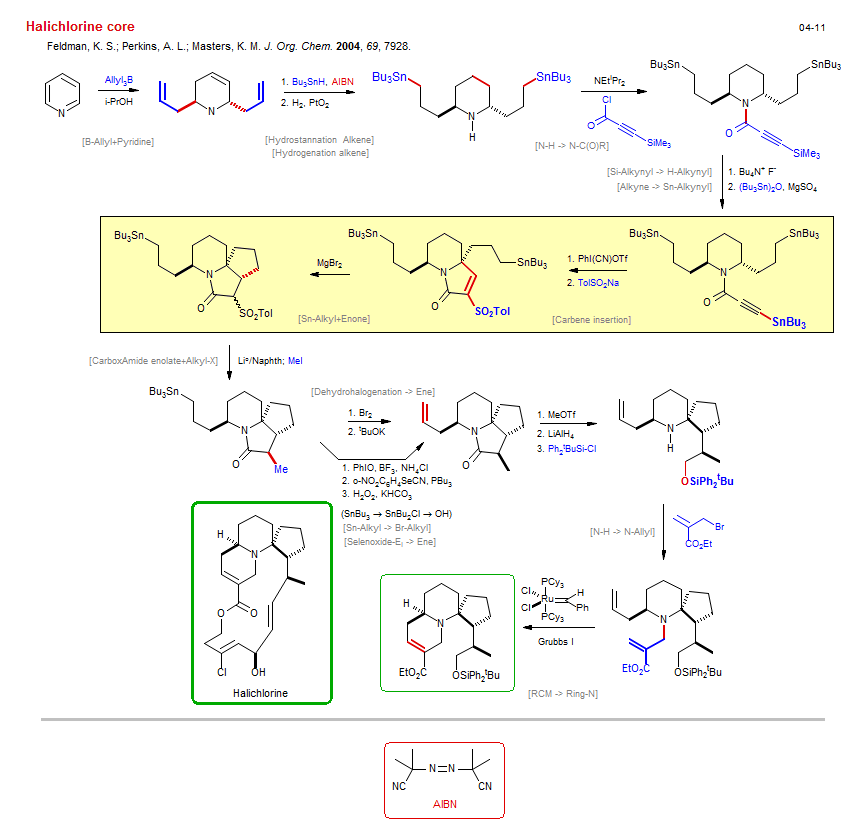
Total synthesis of Halichlorine core:
| Reference: | Feldman, K. S.; Perkins, A. L.; Masters, K. M. J. Org. Chem. 2004, 69, 7928. DOI |
| Reactions: | Iodonium • Alkynyliodonium • Ketone-α-S(O)nR → Ketone • Sn-Alkyl → Br-Alkyl • Selenide → Selenoxide • Alkyl-X → Alkyl-SeR • CarboxAmide → Alkyl-OH • O-H → O-SiPh2tBu • RCM → Ring-N • Amine-6 • Bicyclo(440)-aza • B-Allyl+Pyridine • Hydrostannation Alkene • Selenoxide-Ei → Ene • Sn-Alkyl+Enone • Enone+Sn-R • ConjAdd Sn-R • Cyclopentane • Bicyclo(330)-aza • Hydrogenation alkene • N-H → N-Allyl • N-H → N-C(O)R • Dehydrohalogenation → Ene • Sn-Alkyl → Br-Alkyl • Carbene insertion • Lactam-5 • Bicyclo(430)-aza • CarboxAmide enolate+Alkyl-X • Alkyl-X+Enolate • Si-Alkynyl → H-Alkynyl • Alkyne → Sn-Alkynyl • |
| Reagents: | Allyl-BR2 • HSnBu3 • H2, Pt • Alkyl-SnR3 • Alkyl-SnR3 • Alkynyl-SiR3 • NEtiPr2 • Alkynyl-SnR3 • Fluoride, R4N+ • SnBu3-OSnBu3 • Iodosobenzene-cyanotriflate • Sulfinate, toluene • MgBr2 • Li°/Naphth • Br2 • KOtBu • Iodosobenzene • H2O2, KHCO3 • Selenocyanate, o-nitrophenyl • MeOTf • AlH4-Li+ • Allyl-Br • Acrylate • SiPh2tBu-Cl • Grubbs I • AIBN • BF3 • PBu3 • |