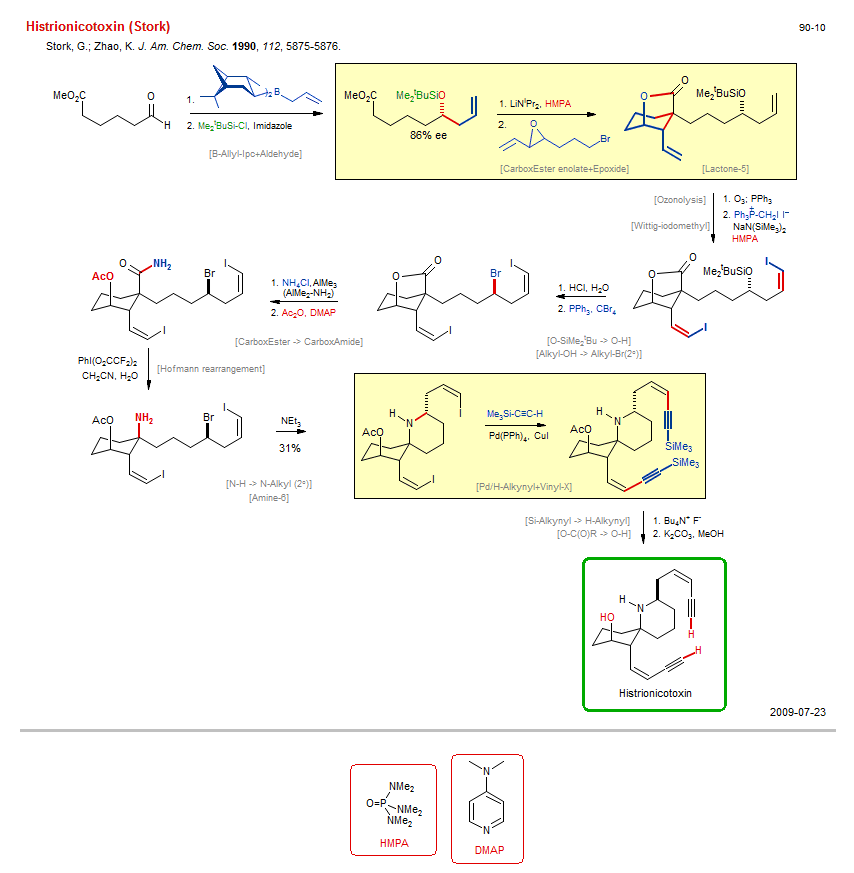
Total synthesis of Histrionicotoxin (Stork):
| Reference: | Stork, G.; Zhao, K. J. Am. Chem. Soc. 1990, 112, 5875-5876. DOI |
| Reactions: | O-H → O-SiMe2tBu • O-H → O-C(O)R • B-Allyl-Ipc+Aldehyde • Aldehyde+B-Allyl (asym) • CarboxEster enolate+Epoxide • Epoxide+Enolate • CarboxEster enolate+Alkyl-X (intram) • Lactone-5 • Lactone-5 • Mechanism • Ozonolysis • Wittig-iodomethyl • Alkyl-OH → Alkyl-Br(2°) • Appel → Br • Hofmann rearrangement • C-CO2H → C-N • Pd/H-Alkynyl+Vinyl-X • Si-Alkynyl → H-Alkynyl • N-H → N-Alkyl (2°) • CarboxEster → CarboxAmide • O-SiMe2tBu → O-H • O-C(O)R → O-H • Amine-6 • Spiro(44)-aza • |
| Reagents: | Imidazole • O3; PPh3 • PPh3=CH-I • NaN(SiMe3)2 • HMPA (solvent) • HCl, H2O • PPh3, CBr4 • Chloride, ammonium • AlMe3 • Acetic anhydride, DMAP • NEt3 • Alkynyl-SiR3 • CuI • Fluoride, R4N+ • Carbonate, potassium • Chiral auxiliary (boronate) • Allyl-B(Ipc)2 • LiNiPr2, HMPA • Epoxide • HMPA (solvent) • AlMe2-NR2 • |