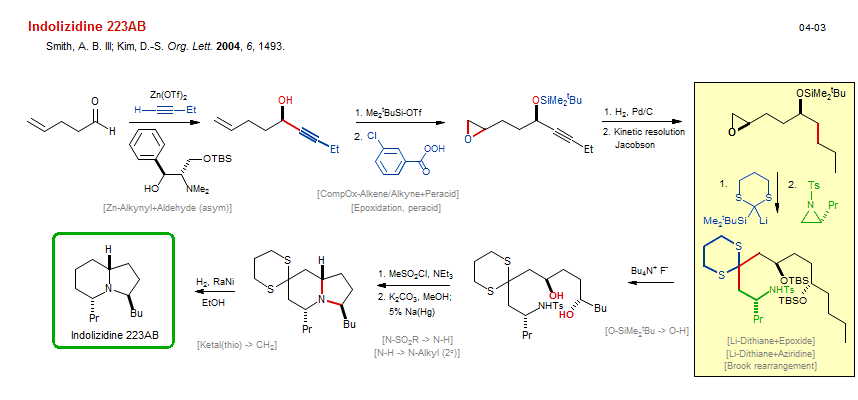
Total synthesis of Indolizidine 223AB:
| Reference: | Smith, A. B. III; Kim, D.-S. Org. Lett. 2004, 6, 1493. DOI |
| Reactions: | O-H → O-SiMe2tBu • Alkyne → Alkane • Jacobson kinetic resolution • O-H → O-SO2Me • Zn-Alkynyl+Aldehyde (asym) • Aldehyde+Zn-R • N-SO2R → N-H • Bicyclo(430)-aza • CompOx-Alkene/Alkyne+Peracid • Epoxidation, peracid • CompOx-Alkene/Alkyne+Peracid • N-H → N-Alkyl (2°) • Amine-5 • Amine-6 • Bicyclo(430)-aza • Li-Dithiane+Epoxide • Li-Dithiane+Aziridine • Ketal(thio) → CH2 • Hydrogenolysis C-S • O-SiMe2tBu → O-H • Brook rearrangement • |
| Reagents: | Zn(OTf)2 • Chiral catalyst (amino-alcohol) • Alkynyl-ZnX • SiMe2tBu-OTf • H2, Pd • Sulfonyl chloride, methane, NEt3 • Carbonate, potassium • H2, RaNi • Na(Hg) • Epoxide • Aziridine • Fluoride, R4N+ • Perbenzoic acid, 3-chloro • |