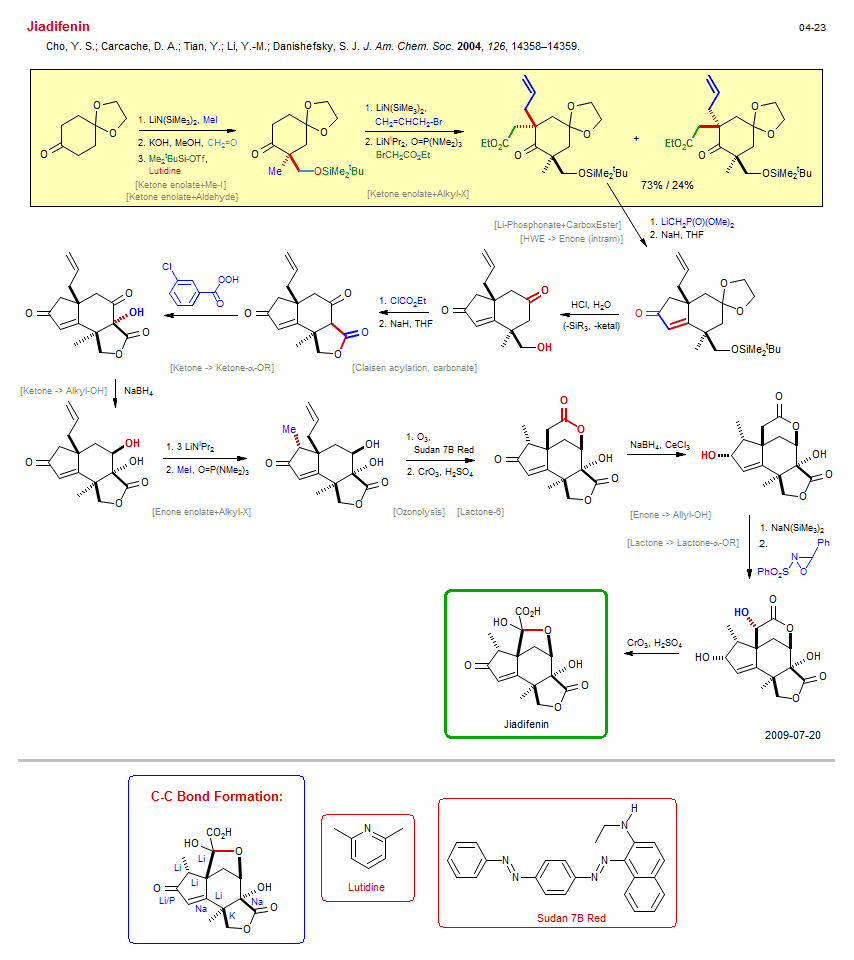
Total synthesis of Jiadifenin:
| Reference: | Cho, Y. S.; Carcache, D. A.; Tian, Y.; Li, Y.-M.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 14358–14359. DOI |
| Reactions: | Enone → Allyl-OH • CompRed-Enone/Lactone+M-H • O-H → O-SiMe2tBu • Ketone enolate+Me-I • Ketone enolate+Aldehyde • Aldol-Na → Aldol • Ketone enolate+Alkyl-X • Bromoacetate+Enolate • HWE → Enone (intram) • Cyclopentenone • Bicyclo(430) • O-H → O-C(O)OR • Lactone-5 • Claisen acylation, carbonate • Ketone enolate+Carbonate • Lactone-5 • Ketone → Ketone-α-OR • Ketal → Ketone • O-SiMe2tBu → O-H • Aldehyde → CarboxAcid • Lactone-6 • Lactone-6 • Enone enolate+Alkyl-X • Alkyl-X+Enolate • Ozonolysis • CompOx-Alkene/Enone+O3 • Lactone-6 • Ketone → Alkyl-OH • CompRed-Ketone/Lactone+M-H • CompRed-Ketone/Enone+M-H • CompE+-Lactone/Lactone+Base • Allyl-OH → Enone • Lactone → Lactone-α-OR • Oxaziridine+Enolate • Li-Phosphonate+CarboxEster • CompE+-CarboxEster/Ketone+M-R • |
| Reagents: | LiN(SiMe3)2 • MeI • Hydroxide, potassium • Formaldehyde • SiMe2tBu-OTf • Lutidine • LiN(SiMe3)2 • Allyl-Br • LiNiPr2 • Bromoacetate • LiCH2-PO(OMe)2 • NaH • HCl, H2O • O=C(OEt)Cl • NaH • Perbenzoic acid, 3-chloro • BH4-Na+ • LiNiPr2 • MeI • O3 • Sudan 7B Red • CrO3, H2SO4 (Jones) • BH4-Na+, CeCl3 • HMPA (solvent) • NaN(SiMe3)2 • Oxaziridine • CrO3, H2SO4 (Jones) • |