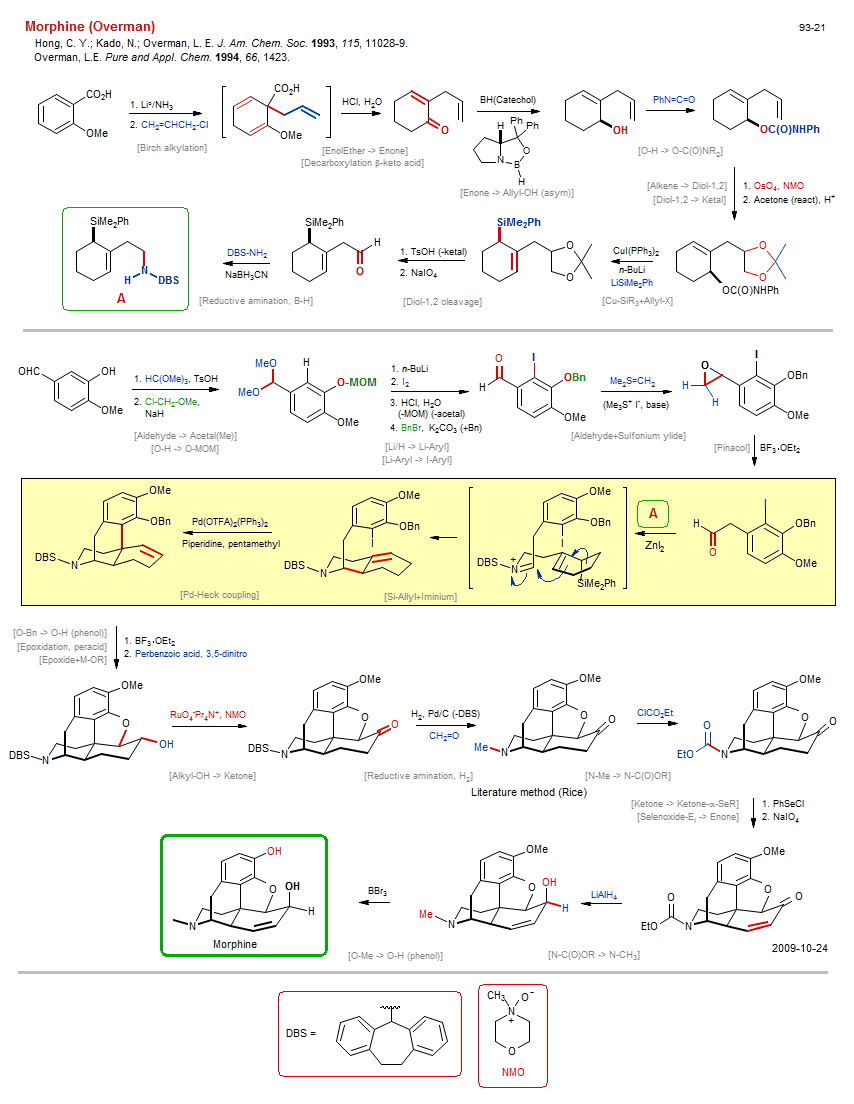
Total synthesis of Morphine (Overman):
| Reference: | Hong, C. Y.; Kado, N.; Overman, L. E. J. Am. Chem. Soc. 1993, 115, 11028-9. DOI |
| Reactions: | CompRed-Enone/Alkene+M-H • Ketal → Diol-1,2 • Epoxide → Diol • Ether-5 • N-Bn → N-H • Birch alkylation • CarboxAcid enolate+Alkyl-X • Allyl-X+Enolate • Alkene → Diol-1,2 • Cu-SiR3+Allyl-X • Allyl-X+Cu-R • Reductive amination, B-H • Aldehyde → Acetal(Me) • Li/H → Li-Aryl • Aldehyde+Sulfonium ylide • Epoxidation ylide • Corey-Chaykowsky • Aldehyde → Epoxide • Pinacol • Reductive amination, H2 • Epoxidation, peracid • Si-Allyl+Iminium • Iminium+Si-R • Amine-6 • Bicyclo(440)-aza • Pd-Heck coupling • Cyclohexane • Bicyclo(321) • Bicyclo(330)-aza • O-H → O-Alkyl (phenol) • EnolEther → Enone • Decarboxylation β-keto acid • O-H → O-C(O)NR2 • Diol-1,2 → Ketal • Diol-1,2 cleavage • Alkene cleavage • Epoxide+M-OR • O-Bn → O-H (phenol) • Alkyl-OH → Ketone • Li-Aryl → I-Aryl • O-H → O-MOM • O-MOM → O-H • Acetal → Aldehyde • N-Me → N-C(O)OR • Ketone → Ketone-α-SeR • Selenoxide-Ei → Enone • Selenide → Selenoxide • N-C(O)OR → N-CH3 • O-Me → O-H (phenol) • Enone → Allyl-OH (asym) • |
| Reagents: | Li°/NH3 • Allyl-Cl • HCl, H2O • BH(Catechol) • Chiral catalyst (amino-alcohol) • Corey oxazaborolidine • Isocyanate • CuI • BuLi(n) • SiR3-Li • OsO4, NMO • Acetone (react) • TsOH • Periodate, sodium • BH3CN-Na+ • Orthoformate • Chloromethyl ether • BuLi(n) • I2 • HCl, H2O • Me3S+ I- • BF3·OEt2 • Benzyl-X • BF3·OEt2 • Perbenzoic acid, 3,5-dinitro • RuO4-Pr4N+ • H2, Pd • Formaldehyde • O=C(OEt)Cl • SePh-Cl • Periodate, sodium • AlH4-Li+ • B(Br)3 • Pd(OTFA)2(PPh3)2 • Piperidine, pentamethyl • Carbonate, potassium • Aryl-Li • Me2S=CH2 • NaH • |